Abstract
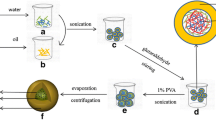
Core-shell nanoparticulate formulation of gemcitabine was prepared by incorporating gemcitabine in a hydrophilic bovine serum albumin (BSA) core surrounded by hydrophobic poly(dl-lactic acid-co-glycolic acid) (PLGA) shell with a particle size of 243 nm and encapsulation efficiency of 40.5 %. Prepared formulations were lyophilized, wherein several cryoprotectants were screened for product attributes such as cake appearance, reconstitution with water, and size constancy. Trehalose was screened as a lyoprotectant, which showed stability for 6 months at 5 °C and 25 °C/60 % relative humidity (RH) conditions. However, an increase in particle size was observed at accelerated conditions (40 °C/75 % RH). In vitro evaluation of these nano-formulations in MCF-7 breast cancer cells showed enhanced cellular uptake (90 %) as compared to GEMCITE® uptake (51 %) in 6 h along with reduced IC50 value at 72 h (16 μM versus 30 μM). In vivo studies in Sprague Dawley rats showed C max, t 1/2, and area under the curve (AUC) at 2.55 μg/ml, 13.6 h, and 28,322.5 μg/l/h, respectively, whereas GEMCITE® at the same dose showed significantly lower corresponding values at 1.94 μg/ml, 6.89 h, and 13,967 μg/l/h. In the same study, AUC and C max of inactive metabolite of gemcitabine (dFdU) were reduced by 33 and 42 %, respectively, for these nanoparticles compared to GEMCITE®. In 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer model, significantly reduced tumor growth was observed in gemcitabine-loaded-nanoparticle-treated animals compared with GEMCITE®-treated animal at equivalent dose (121 versus 243 % in 30 days). The results indicated that our core-shell nanoparticles are more effective for tumor reduction compared to marketed formulation of gemcitabine, GEMCITE®.





Similar content being viewed by others
References
Reddy LH, Couvreur C. Novel approaches to deliver gemcitabine to cancer. Curr Pharm Design. 2008;14:1124–37.
Bornmann C, Graeser R, Esser N, Ziroli V, Jantscheff P, Keck T, et al. A new liposomal formulation of gemcitabine is active in an orthotopic mouse model of pancreatic cancer accessible to bioluminescence imaging. Cancer Chemother Pharmacol. 2008;61:395–405.
Calvagno MG, Celia C, Paolino D, Cosco D, Iannone M, Castelli F, et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr Drug Deliv. 2007;4:89–101.
Celano M, Cavalgno MG, Bulotta S, Paolino D, Arturi F. Cytotoxic effects of gemcitabine-loaded liposomes in human anaplastic thyroid carcinoma cells. BMC Cancer. 2004;4:63–70.
Celia C, Malara N, Terracciano R, Cosco D, Paolino D, Fresta M, et al. Liposomal delivery improves the growth-inhibitory and apoptotic activity of low doses of gemcitabine in multiple myeloma cancer cells. Nanomed Nanotech Biol Med. 2008;4:155–66.
Moog R, Burger AM, Brandl M, Schuler J, Schubert R, Unger C. Change in pharmacokinetic and pharmacodynamic behavior of gemcitabine in human tumor xenografts upon entrapment in vesicular phospholipid gels. Cancer Chemother Pharmacol. 2002;49:356–66.
Papa AL, Basu S, Sengupta P, Banerjee D, Sengupta S, Harfouche R. Mechanistic studies of Gemcitabine-loaded nanoplatforms in resistant pancreatic cancer cells. BMC Cancer. 2012;12:419.
Aggarwal S, Yadav S, Gupta S. EGFR targeted PLGA nanoparticles using gemcitabine for treatment of pancreatic cancer. J Biomed Nanotechnol. 2011;7:137–8.
Gang J, Park S, Hyung W, Choi EH, Wen J, Kim H, et al. Magnetic poly caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model. J Drug Target. 2007;15:445–53.
Li J, Chen W, Wang H, Jin C, Yu X, Lu W, et al. Preparation of albumin nanospheres loaded with gemcitabine and their cytotoxicity against BXPC-3 cells in vitro. Acta Pharmacol Sin. 2009;30:1337–43.
Yang J, Lee H, Hyung W, Park S, Haam S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: synthesis and release characteristics. J Microencapsul. 2006;23:203–12.
Yang J, Park S, Yoon H, Huh Y, Haam S. Preparation of poly caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int J Pharm. 2006;324:185–90.
Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970–8.
Xu J, Singh A, Amiji MM. Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer. BMC Cancer 2014;14:75-2407-14-75.
Singh R, Mehra NK, Jain V, Jain NK. Gemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cells. J Drug Target. 2013;21:581–92.
Gupta A, Asthana S, Konwar R, Chourasia MK. An insight into potential of nanoparticles-assisted chemotherapy of cancer using gemcitabine and its fatty acid prodrug: a comparative study. J Biomed Nanotechnol. 2013;9:915–25.
Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of gemcitabine conjugated and miR-205 cmplexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35:7077–87.
Chitkara D, Mittal A, Behrman SW, Kumar N, Mahato RI. Self-assembling, amphiphilic polymer-gemcitabine conjugate shows enhanced antitumor efficacy against human pancreatic adenocarcinoma. Bioconjug Chem. 2013;24:1161–73.
Cavallaro G, Mariano L, Salmaso S, Caliceti P, Gaetano G. Folate-mediated targeting of polymeric conjugates of gemcitabine. Int J Pharm. 2006;307:258–69.
Arias JL, Reddy LH, Couvreur P. Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J Drug Target. 2009;17:586–98.
Paolino D, Cosco D, Celano M, Moretti S, Puxeddu E, Russo D, et al. Gemcitabine-loaded biocompatible nanocapsules for the effective treatment of human cancer. Nanomedicine (Lond). 2013;8:193–201.
Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7:1851–63.
Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 2011;7:859–70.
Derakhshandeh K, Fathi S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int J Pharm. 2012;437:172–7.
You J, Liu Y, Peng C. Efficient gene transfection using chitosan–alginate core-shell nanoparticles. Int J Nanomedicine. 2006;1:173–80.
Martin-Banderas L, Saez-Fernandez E, Holgado MA, Duran-Lobato MM, Prados JC, Melguizo C, et al. Biocompatible gemcitabine-based nanomedicine engineered by Flow Focusing((R)) for efficient antitumor activity. Int J Pharm. 2013;443:103–9.
Chitkara D, Kumar N. BSA-PLGA-based core-shell nanoparticles as carrier system for water-soluble drugs. Pharm Res. 2013;30:2396–409.
Chitkara D, Nikalaje SK, Mittal A, Chand M, Kumar N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Dev Trans Res. 2012;2:112–23.
Ayen WY, Kumar N. A systematic study on lyophilization process of polymersomes for long-term storage using doxorubicin-loaded (PEG)(3)-PLA nanopolymersomes. Eur J Pharm Sci. 2012;46:405–14.
Uckun FM, Dibirdik I, Qazi S, Vassilev A, Ma H, Mao C, et al. Anti-breast cancer activity of LFM-A13, a potent inhibitor of Polo-like kinase (PLK). Bioorg Med Chem. 2007;15:800–14.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Chitkara D, Singh S, Kumar V, Danquah M, Behrman SW, Kumar N, et al. Micellar delivery of cyclopamine and gefitinib for treating pancreatic cancer. Mol Pharm. 2012;9:2350–7.
Lee MK, Kim MY, Kim S, Lee J. Cryoprotectants for freeze drying of drug nano-suspensions: effect of freezing rate. J Pharm Sci. 2009;98:4808–17.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–713.
Ahlin P, Kristl J, Kristl A, Vrecer F. Investigation of polymeric nanoparticles as carriers of enalaprilat for oral administration. Int J Pharm. 2002;239:113–20.
De Jaeghere F, Allemann E, Leroux JC, Stevels W, Feijen J, Doelker E, et al. Formulation and lyoprotection of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res. 1999;16:859–66.
Kim MJ, Lee HJ, Lee IA, Kim IY, Lim SK, Cho HA, et al. Preparation of pH-sensitive, long-circulating and EGFR-targeted immunoliposomes. Arch Pharm Res. 2008;31:539–46.
dos Santos T, Varela J, Lynch I, Salvati A, Dawson KA. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One. 2011;6:e24438.
Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3′-deoxy-3′-fluorothymidine. Biochem Pharmacol. 2010;79:587–95.
Marce S, Molina-Arcas M, Villamor N, Casado FJ, Campo E, Pastor-Anglada M, et al. Expression of human equilibrative nucleoside transporter 1 (hENT1) and its correlation with gemcitabine uptake and cytotoxicity in mantle cell lymphoma. Haematologica. 2006;91:895–902.
Paolino D, Cosco D, Licciardi M, Giammona G, Fresta M, Cavallaro G. Polyaspartylhydrazide copolymer-based supramolecular vesicular aggregates as delivery devices for anticancer drugs. Biomacromolecules. 2008;9:1117–30.
Dadashzadeh S, Derakhshandeh K, Shirazi FH. 9-nitrocamptothecin polymeric nanoparticles: cytotoxicity and pharmacokinetic studies of lactone and total forms of drug in rats. Anticancer Drugs. 2008;19:805–11.
Reddy LH, Murthy RS. Pharmacokinetics and biodistribution studies of Doxorubicin loaded poly(butyl cyanoacrylate) nanoparticles synthesized by two different techniques. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148:161–6.
Cosco D, Bulotta A, Ventura M, Celia C, Calimeri T, Perri G, et al. In vivo activity of gemcitabine-loaded PEGylated small unilamellar liposomes against pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:1009–20.
Schmid P, Akrivakis K, Flath B, Grosse Y, Sezer O, Mergenthaler HG, et al. Phase II trial of gemcitabine as prolonged infusion in metastatic breast cancer. Anticancer Drugs. 1999;10:625–31.
Acknowledgments
The Department of Biotechnology, India, is gratefully acknowledged for providing financial support. Authors are thankful to the Department of Science and Technology, India, for CLSM and TEM facilities at NIPER, SAS Nagar. The Ranbaxy Science Foundation (RSF), India, is duly acknowledged for recognizing this work for Ranbaxy Science Scholar Award 2011.
Conflict of interest
Authors DC, AM, RIM, and NK declare that they have no conflict of interest.
Animal studies disclosure
All procedures performed in studies involving animals were approved by the Institutional Animal Ethics Committee (IAEC), NIPER, SAS Nagar, India, and were in accordance with the CPCSEA guidelines, India, for the care and use of laboratory animals.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chitkara, D., Mittal, A., Mahato, R.I. et al. Core-shell nanoparticulate formulation of gemcitabine: lyophilization, stability studies, and in vivo evaluation. Drug Deliv. and Transl. Res. 4, 439–451 (2014). https://doi.org/10.1007/s13346-014-0206-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-014-0206-y