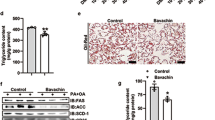
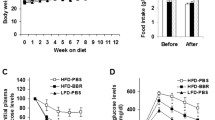
Abstract
Genetic and pharmacological activation of the transcription factor nuclear factor, erythroid derived 2, like 2 (Nrf2) alleviates high-fat diet (HFD)-induced obesity in mice; however, synthetic Nrf2 activators are not clinically available due to safety concerns. Dietary glucoraphanin (GR), a naturally occurring compound found in cruciferous vegetables that activates Nrf2 and induces its target antioxidant genes. We previously demonstrated that GR increased thermogenesis and mitigated HFD-induced obesity in lean healthy mice. In this study, we investigated the therapeutic effects of GR on pre-existing obesity and associated metabolic disorders, such as hepatic steatosis, with or without low-fat dietary intervention. Eight-week-old male C57BL/6J mice were fed an HFD for 9 weeks to induce obesity. Subsequently, these obese mice were fed either the HFD or a normal chow diet, supplemented with or without GR, for an additional 11 weeks. GR supplementation did not decrease the body weight of HFD-fed mice; however, it significantly reduced plasma alanine aminotransferase and aspartate aminotransferase levels and hepatic triglyceride accumulation. These improvements in liver damage by GR were associated with decreased expression levels of fatty acid synthesis genes and proinflammatory chemokine genes, suppressed c-Jun N-terminal kinase activation, and reduced proinflammatory phenotypes of macrophages in the liver. Moreover, metabolome analysis identified increased hepatic levels of adenosine 5′-monophosphate (AMP) in HFD-GR mice compared with those in HFD mice, which agreed with increased phosphorylation levels of AMP-activated protein kinase. Our results show that GR may have a therapeutic potential for treating obesity-associated hepatic steatosis.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–82.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57.
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–9.
Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–203.
Chartoumpekis DV, Kensler TW. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr Diabetes Rev. 2013;9:137–45.
Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33:2996–3010.
Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem. 2010;285:40581–92.
Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, et al. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54:922–34.
de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503.
Kelley MJ, Glaser EM, Herndon JE, Becker F, Bhagat R, Zhang Y-J, et al. Safety and efficacy of weekly oral oltipraz in chronic smokers. Cancer Epidemiol Biomark Prev. 2005;14:892–9.
Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–9.
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. https://doi.org/10.1038/ncomms11624.
Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. 2013;71:709–26.
Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9:eaah4477. https://doi.org/10.1126/scitranslmed.aah4477.
Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes. 2017;66:1222–36.
Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Muñoz A, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33:101–7.
Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–90.
Obstfeld AE, Sugaru E, Thearle M, Francisco A-M, Gayet C, Ginsberg HN, et al. C–C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–25.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–96.
Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25.
Lin T-Y, Cantley LC, DeNicola GM, Lin T-Y, Cantley LC, DeNicola GM. NRF2 rewires cellular metabolism to support the antioxidant response. IntechOpen. 2016. https://doi.org/10.5772/65141.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62.
Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–13.
Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294–303.
Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770–81.
Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96.
Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310-1321.
Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–26.
Patsouris D, Li P-P, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9.
Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507.
Pal S, Konkimalla VB. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int Immunopharmacol. 2016;35:85–98.
Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218.
Lu SC. Glutathione synthesis. Biochim Biophys Acta. 1830;2013:3143–53.
Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–66.
Woods A, Williams JR, Muckett PJ, Mayer FV, Liljevald M, Bohlooly-Y M, et al. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 2017;18:3043–51.
Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–40.
Zhao X, Sun X, Chaggan C, Liao Z, Wong KI, He F, et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 2020;367:652–60.
Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11:655–68.
Yu Y, Cai W, Zhou J, Lu H, Wang Y, Song Y, et al. Anti-arthritis effect of berberine associated with regulating energy metabolism of macrophages through AMPK/ HIF-1α pathway. Int Immunopharmacol. 2020;87:106830. https://doi.org/10.1016/j.intimp.2020.106830.
Cai W, Cheng J, Zong S, Yu Y, Wang Y, Song Y, et al. The glycolysis inhibitor 2-deoxyglucose ameliorates adjuvant-induced arthritis by regulating macrophage polarization in an AMPK-dependent manner. Mol Immunol. 2021;140:186–95.
Petsouki E, Cabrera SNS, Heiss EH. AMPK and NRF2: Interactive players in the same team for cellular homeostasis? Free Radic Biol Med. 2022;190:75–93.
Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, et al. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol. 2014;34:3305–20.
Xu J, Donepudi AC, Moscovitz JE, Slitt AL. Keap1-knockdown decreases fasting-induced fatty liver via altered lipid metabolism and decreased fatty acid mobilization from adipose tissue. PLoS ONE. 2013;8:e79841. https://doi.org/10.1371/journal.pone.0079841.
Kikuchi M, Ushida Y, Shiozawa H, Umeda R, Tsuruya K, Aoki Y, et al. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J Gastroenterol. 2015;21:12457–67.
Acknowledgements
The authors would like to thank Dr. Tsuguhito Ota for kindly supporting this project. The authors thank H Akutsu (Asahikawa Medical University, Asahikawa, Japan) for technical assistance of GC-MS analysis. The authors also thank M Ishiguro and C Hasegawa (Asahikawa Medical University, Asahikawa, Japan), M Nakayama and K Hara (Kanazawa University, Kanazawa, Japan) for technical assistance and the care of animals. Parts of the data in this article were presented at IDF conference December 2021 (IDF21-0354).
Funding
This work was supported by a grant Grant-in-Aid for Scientific Research (C) (15K00813 for N.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Contributions
SP, KS, MN, and LX performed experiments and collected and analyzed data. SP and KS wrote first draft of the manuscript. SP, NN and YT contributed to discussions and edited the manuscript. YT supervised the project. All authors reviewed and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y.T. was supported by several foundational grants, including grants from Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Ono pharmaceutical Co., Ltd. From October 2019 to September 2021, K.S. was belonged to the Division of Diabetes and Lifestyle Diseases Prevention and Therapeutics, an endowed course at Asahikawa Medical University, which was run with donations from Ono pharmaceutical Co., Ltd, Taisho Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. No other potential conflicts of interest relevant to this article were reported.
Compliance with ethical standards
All animal procedures were approved by the Kanazawa University Animal Care and Use Committee (date of approval: September 5, 2013, approved number: AP-132930) and were performed in accordance with the standards set forth in the guidelines and regulations for the Care and Use of Laboratory Animals at Kanazawa University. This study does not contain any studies with human subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Promsuwan, S., Sawamoto, K., Xu, L. et al. A natural Nrf2 activator glucoraphanin improves hepatic steatosis in high-fat diet-induced obese male mice associated with AMPK activation. Diabetol Int 15, 86–98 (2024). https://doi.org/10.1007/s13340-023-00658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-023-00658-6