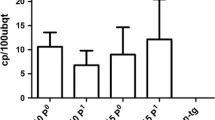
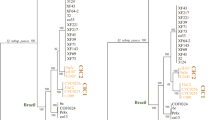
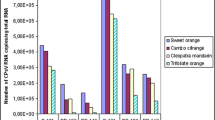
Abstract
Psorosis is a globally devastating disease of citrus caused by an infectious filamentous ophiovirus, Citrus psorosis virus (CPsV), which causes annual losses of about 5 % and a progressive decline of trees by affecting the conductive tissues. The disease can be harboured asymptomatically in many citrus species. In the field, the most characteristic symptoms of the disease in adult trees are bark scaling in the trunk and main branches and also internal staining in the underlying wood. The virus has a tripartite single-stranded RNA genome, and has been inadvertently spread to most citrus growing areas through the movement of citrus propagative material. No natural vectors have been identified except in limited citrus areas in some cases. Management strategies for CPsV involving shoot-tip grafting and thermotherapy or somatic embryogenesis from stigma and style cultures have been successfully used to eliminate CPsV from plant propagating material. Molecular pathogen-mediated strategies have been used to produce citrus plants. Such a strategy protects against infections by the virus from which the resistance gene and promising resistance may emerge from trials. Certification programs are among the best established means of increasing phytosanitary health, and some of those for citrus are among the oldest in the world. In conjunction with quarantine and clean stock programs, they remain important weapons in the ongoing fight against citrus diseases. One of the elements essential for successful certification programs to produce such propagation material is the availability of sensitive and effective diagnostic methods. In this review, we discuss an updated status of CPsV disease.

Similar content being viewed by others
References
Alioto D, Gangemi M, Deaglio S, Sposato S, Noris E, Luisoni E, Milne RG. Improved detection of Citrus psorosis virus using polyclonal and monoclonal antibodies. Plant Pathol. 1999;48:735–41.
Alioto D, Troisi A, Peluso A, Quatrano G, Masenga V, Milne RG. Occurence of Citrus psorosis virus in Campania, Southern Italy. Eur J Plant Pathol. 2000;106:795–9.
Alioto D, Malfitano M, Troisi A, Peluso A, Martín S, Milne RG, Guerri J, Moreno P. Variability of the coat protein gene of Citrus psorosis virus in Campania, southern Italy. Arch Virol. 2003;148:2155–66.
Barragan-Valencia G, Morales-Loredo A, Alvarez-Ojeda MG, Rio MAPD, Quintero-Zapata I. Quantitative diagnosis of Citrus psorosis virus by real time RT-PCR. Agrociencia. 2008;42(2):225–32.
Barthe GA, Ceccardi GL, Manjunath KL, Derrick KS. Citrus psorosis virus: nucleotide sequencing of the coat protein gene and detection by hybridization and RT-PCR. J Gen Virol. 1998;79:1531–7.
Carvalho SA, Santos FA, Machado MA. Psorosis virus complex elimination from citrus by shoot-tip-grafting associated to thermotherapy. Fitopatologia Brasileira. 2002;27(3):306–8.
Da Graça JV, Bar-Joseph M, Derrick KS. Inmunoblot detection of citrus psorosis in Israel using citrus ringspot antiserum. Proceedings of the 12th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 1993, pp 432–434.
Danos E. La psorosis de los cítricos: la epidemia en curso en Argentina y el desafio de su control. Revista de Investigaciones Agropecuarias. 1990;22:265–77.
Derrick KS, Brlansky RH, da Graça JV, Lee RF, Timmer LW, Nguyen TK. Partial characterization of a virus associated with citrus ringspot. Phytopathology. 1988;78:1298–301.
Derrick KS, Lee RF, Hewitt BG, Barthe GA, da Graça JV. Characterization of citrus ringspot virus. In: Timmer LW, Garnsey SM, Navarro L, editors. Proceedings of the 11th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 1991, pp 386–390.
Djelouah K, Potere O, Boscia D, D’Onghia AM, Savino V. Production of monoclonal antibodies to citrus psorosis associated virus. In: da Graça JV, Lee RF, Yokomi RH, editors. Proceedings of the 14th International Organization of Citrus Virologists (IOCV) Conference, Riverside. 2000, pp 152–158.
D’Onghia AM, Djelouah K, Alioto KM, Castellano A, Savino V. Elisa correlates with biological indexing for the detection of Citrus psorosis virus associated virus. J Plant Pathol. 1998;80:157–63.
D’Onghia AM, Djelouah K, Savino V. Serological detection of Citrus psorosis virus in seeds but not in seedlings of infected mandarin and sour orange. J Plant Pathol. 2000;82(3):233–5.
D’Onghia AM, Carimi F, De Pasquale F, Djelouah K, Martelli GP. Elimination of Citrus psorosis virus by somatic embryogenesis from stigma and style cultures. Plant Pathol. 2001;50(2):266–9.
D’Onghia AM, Djelouah K, Frasheri D, Potere O. Detection of Citrus psorosis virus by direct tissue blot immunoassay. J Plant Pathol. 2001;83:139–42.
EPPO. Data Sheets on Quarantine Pests: Citrus ringspot virus. Prepared by CABI and EPPO for the EU under Contract 90/399003. 2005. http://www.eppo.org/QUARANTINE/virus/Citrus_ringspot_virus/CIRSV0_ds.pdf.
Febres V, Fisher L, Khalaf A, Moore AG. Citrus transformation: challenges and prospects. Genetic transformation. 2011;101–22.
Garçia ML, Grau O, Sarachu AN. Citrus psorosis is probably caused by a bipartite ssRNA virus. Res Virol. 1991;142:303–11.
Garçia ML, Dal Bó E, Grau O, Milne RG. The closely related citrus ringspot and Citrus psorosis viruses have particles of novel filamentous morphology. J Gen Virol. 1994;75:3585–90.
Garçia ML, Sánchez de la Torre ME, Dal Bó E, Djelouah K, Luisoni E, Milne RG, Grau O. Detection of citrus psorosis-ringspot using RT-PCR and ELISA. Plant Pathol. 1997; 46:830–6.
Gottwald TR, Palle SR, Miao H, Seyran M, Skaria M, da Graça JV. Assessment of the possibility of natural spread of citrus psorosis disease. Proceedings of the 16th International Organization of Citrus Virologists (IOCV) Conference, Riverside. 2005, pp 240–250.
Kawazu Y, Sasaya T, Morikawa T, Sugiyama K, Natsuaki T. Nucleotide sequence of the coat protein gene of Mirafiori lettuce virus. J Gen Plant Pathol. 2003;69:55–60.
Kayim M, Barthe G, Beretta J, Derrick K. Transgenic citrus plants expressing the coat protein gene of Citrus psorosis virus. Phytopathology. 2005;95(6):S52–S52 Suppl.
Khan IA. Citrus genetics, breeding and biotechnology. Cambridge: CABI Wallingford; 2007.
Lee RF, Lehmann P, Navarro L. Nursery practices, budwood and rootstock certification programs. In: Timmer LW, Duncan L, editors. Citrus health guide. St. Paul: APS Press Minn; 1999. p. 35–46.
Legarreta GG, Garcia ML, Costa N, Grau O. A highly sensitive heminested RT-PCR assay for the detection of Citrus psorosis virus targeted to a conserved region of the genome. J Virol Methods. 2000;84:15–22.
Loconsole G, Castellano MA, Dell’Orco M, Boscia D, Savino V. Serological detection of Citrus psorosis virus using a polyclonal antiserum to recombinant virus coat protein. J Plant Pathol. 2006;88:171–3.
Loconsole G, Fatone MT, Savino V. Specific digoxigenin-labeled riboprobes for detection of Citrus prsorosis virus and Citrus variegation virus by molecular hybridization. J Plant Pathol. 2009;91(2):311–9.
Loconsole G, Saponari M, Savino V. Development of real-time PCR based assays for simultaneous and improved detection of citrus viruses. Eur J Plant Pathol. 2010;128:251–9.
Lot H, Campbell RN, Couche S, Milne RG, Roggero P. Transmisison by Olpidium brassicae of Mirafiori lettuce virus and Lettuce big-vein virus and their roles in lettuce big-vein etiology. Phytopathology. 2002;92:288–93.
Luna GR, Peña EJ, Borniego MB, Heinlein M, Garcia ML. Ophioviruses CPsV and MiLBVV movement protein is encoded in RNA 2 and interacts with the coat protein. Virology. 2013;441(2):152–61.
Martín S, Alioto D, Milne RG, Guerri J, Moreno P. Detection of Citrus psorosis virus in field trees by direct tissue immunoassay in comparison with ELISA, symptomatology, biological indexing and cross-protection tests. Plant Pathol. 2002;51:134–41.
Martín S, Alioto D, Milne RG, Garnsey SM, García ML, Grau O. Detection of Citrus psorosis virus by ELISA, molecular hybridization, RT-PCR and immunosorbent electron microscopy and its association with citrus psorosis disease. Eur J Plant Pathol. 2004;110:747–57.
Martín S, López C, García ML, Naum-Onganía G, Grau O, Flores R. The complete nucleotide sequence of a Spanish isolate of Citrus psorosis virus: comparative analysis with other ophioviruses. Arch Virol. 2005;150(1):167–76.
Martín S, García ML, Troisi A, Rubio L, Legarreta G, Grau O, Alioto D, Moreno P, Guerri J. Genetic variation of populations of Citrus psorosis virus. J Gen Virol. 2006;87:3097–102.
Milne RG, Djelouah K, Garçia ML, Dal Bo E, Grau O. In: Structure of citrus-ringspot psorosis-associated virus particles: implication for diagnosis and taxonomy. Proceedings of the 13th International Organization of Citrus Virologists (IOCV) Conference, China. 1996, pp 189–97.
Milne RG, García ML, Moreno P. Citrus psorosis virus. In: AAB descriptions of plant viruses. 2003. No. 401.
Morikawa T, Nomura Y, Yamamoto T, Natsuaki T. Partial characterization of virus-like particles associated with Tulip mild mottle mosaic. Ann Phytopathol Soc Jpn. 1995;61:78–581.
Naum-Onganía G, Gago-Zachert S, Peña E, Grau O, García ML. Citrus psorosis virus RNA 1 is of negative polarity and potentially encodes in its complementary strand a 24K protein of unknown function and 280K putative RNA dependent RNA polymerase. Virus Res. 2003;96:49–61.
Navarro JA, Torok VA, Vetten HJ, Pallas V. Genetic variability in the coat protein genes of Lettuce big-vein associated virus and Mirafiori lettuce big-vein virus. Arch Virol. 2005;150:681–94.
Navarro L. Citrus sanitation, quarantine and certification programs. Moreno P, da Graça JV, Timmer LW, editors. Proceedings of the 12th International Organization of Citrus Virologists (IOCV) Conference, Riverside. 1993, pp 383–91.
Navas-Castillo J, Moreno P. Biological diversity of citrus ringspot isolates in Spain. Plant Pathol. 1993;42:347–57.
Navas-Castillo J, Moreno P. Filamentous flexuous particles and serologically related proteins of variable size associated with citrus psorosis and ringspot diseases. Eur J Plant Pathol. 1995;101:343–8.
Palle SR, Miao H, Seyran M, Louzada ES, da Graça JV, Skaria M. Evidence for association of Citrus psorosis virus with symptomatic trees and an Olpidium-like fungus. Proceedings of the 14th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 2005, pp 423–6.
Peña E, Luna GR, Zanek MC, Garçia ML. Citrus psorosis virus: insights into virus biology. Proceedings of the 18th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 2010, p 52.
Potere O, Boscia D, Djelouah K, Elicio V, Savino V. Use of monoclonal antibodies to Citrus psorosis virus for diagnosis. J Plant Pathol. 1999;81:209–12.
Powell CA, Pelosi RR, Sodona RM. A psorosis like agent prevalent is Florida’s Grapefruit groves and budwood sources. Plant Dis. 1998;82(2):208–9.
Reyes CA, Peńa EJ, Zanek MC, Sanchez DV, Grau O, García ML. Differential resistance to Citrus psorosis virus in transgenic Nicotiana benthamiana plants expressing hairpin RNA derived from the coat protein and 54K protein genes. Plant Cell Rep. 2009;28:1817–25.
Reyes CA, De Francesco A, Pena EJ, Costa N, Plata MI, Sendin L, Castagnaro AP, Garcia ML. Resistance to Citrus psorosis virus in transgenic sweet orange plants is triggered by coat protein-RNA silencing. J Biotech. 2011;151(1):151–8.
Reyes CA, Zanek MC, Velázquez K, Costa N, Plata MI, Garçia ML. Generation of sweet orange transgenic lines and evaluation of citrus psorosis virus-derived resistance against psorosis A and psorosis B. J Phytopathol. 2011;159:531–7.
Rochon D, Kakani K, Robbins M, Reade R. Molecular aspectes of plant virus transmission by Olpidium and Plasmodiophorid vectors. Annu Rev Phytopathol. 2004;42:211–41.
Roggero P, Ciuffo M, Vaira AM, Accotto GP, Masenga V, Milne RG. An Ophiovirus isolated from lettuce with big-vein symptoms. Arch Virol. 2000;145:2629–42.
Roistacher CN. Graft-transmissible diseases of citrus. Handbook for detection and diagnosis. Rome: Food and Agriculture Organization of the United Nations (FAO); 1991. p. 115–26.
Roistacher CN. Psorosis-A Review. In: Moreno P, da Graça JV, Timmer LW, editors. Proceedings of the 12th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 1993, pp 139–54.
Rosa C, Polek M, Falk BW, Rowhani A. Improved efficiency for quantitative and qualitative indexing for Citrus tristeza virus and Citrus psorosis virus. Plant Dis. 2007;91:1089–95.
Rouag N, Kichi A, Lousini E, Melin R. Etude comparative des relations sérologiques, morphologiques et moléculaires entre le Citrus Ringspot et le Citrus psorosis virus. In: Rapport du 9ème Congrès Arabe pour la Protection des Végétaux, Damas, Syrie. 2006.
Roy A, Fayad A, Barthe G, Brlansky RH. A multiplex polymerase chain reaction method for reliable, sensitive and simultaneous detection of multiple viruses in citrus trees. J Virol Methods. 2005;129:47–55.
Sánchez de la Torre ME, Riva O, Zandomeni R, Grau O, Garcia ML. The top component of Citrus psorosis virus contains two ssRNAs, the smaller encodes the coat protein. Mol Plant Pathol. 1998. http://www.bspp.org.uk/mppol/1998/1019sanchez.
Sánchez de la Torre ME, López C, Grau O, Garcia ML. RNA 2 of citrus psorosis virus is of negative polarity and contains a single open reading frame in its complementary strand. J Gen Virol. 2002;83:1777–81.
Sasaya T, Koganezawa H. Molecular analysis and virus transmission tests place Olpidium virulentus, a vector of Mirafiori lettuce big-vein virus and tobacco stunt virus, as a distinct species rather than a strain of Olpidium brassicae. J Gen Plant Pathol. 2006;72:20–5.
Skaria M, Miao H, Avila E. Post-freeze status of Citrus psorosis virus in Texas. Proceedings of the 15th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. 2002, pp 366–7.
Sofy AR, Moussa AA, Fahmy H, Ghazal SA, El-Dougdoug KA. Anatomical and ultrastructural changes in Citrus leaves infected with Citrus psorosis virus Egyptian isolates. J Appl Sci Res. 2007;3(6):485–94.
Torok VA and Vetten HJ. Characterization of an ophiovirus associated with lettuce ring necrosis. Joint Conference International Working Groups on Legume and Vegetable Viruses (Abstract), Bonn. 2002, p 4.
Torok VA and Vetten HJ. Identification and molecular characterization of a new ophiovirus associated with lettuce ring necrosis disease. Proceedings of Arbeitskreis Viruskrankheiten der Pflanzen, Heidelberg, Germany. 2003.
Torok VA, Vetten HI. Ophiovirus associated with lettuce ring necrosis (Abstract). Cernobbio: European Society for Virology Meeting; 2010. p. 282.
Vaira AM, Milne RG, Accotto GP, Luisoni E, Masenga V, Lisa V. Partial characterization of a new virus from ranunculus with a divided RNA genome and circular supercoiled thread-like particles. Arch Virol. 1997;142:2131–46.
Vaira AM, Accotto GP, Costantini A, Milne RG. The partial sequence of RNA1 of the ophiovirus Ranunculus white mottle virus indicates its relationship to rhabdovirus and provides candidate primers for an ophiovirus-specific RT-PCR test. Arch Virol. 2003;148:1037–50.
Vaira AM, Lisa V, Costantini A, Masenga V, Rapetti S, Milne RG. Ophioviruses infecting ornamentals and a probable new species associated with a severe disease in freesia. Acta Hort. 2006;722:191–9.
Vaira AM, Kleynhans R, Hammond J. First report of Freesia sneak virus infecting Lachenalia cultivars in South Africa. Plant Dis. 2007;91(6):770.
Vaira AM, Hammond J. An update on Freesia sneak virus, a new species in the Ophiovirus genus. 12th ISVDOP, Haarlem, The Netherlands. 2008.
Vaira AM, Milne RG. Ophiovirus. In: Mahy BWJ, Van Regenmortel M, editors. Encyclopedia of Virology. Oxford: Elsevier; 2008. p. 447–54.
Vaira AM, Hansen MA, Murphy C, Reinsel MD, Hammond J. First report of Freesia sneak virus in Freesia sp. in Virginia. Plant Dis. 2009;93(9):965.
Vaira AM, Gago-Zachert S, García ML, Guerri J, Hammond J, Milne RG, Moreno P, Morikawa T, Natsuaki T, Navarro JA, Pallás V, Torok V, Verbeek M, Vetten HJ. Ophioviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. 2011. p. 743–8.
Van der Wilk F, Dullemans AM, Verbeek M, van den Heuvel JM. Nucleotide sequence and genomic organization of an Ophiovirus associated with lettuce bug-vein disease. J Gen Virol. 2002;83:2869–77.
Vapnek J. Legislatively establishing a health certification programme for Citrus. FAO legal papers online. 2009. http://www.fao.org/lega/prs-o1/1po81.pdf.
Velázquez K, Renovell M, Comellas P, Serra M, García ML, Pina JA, Navarro L, Moreno P, Guerri J. Effect of temperature on RNA silencing of a negative stranded RNA plant virus: Citrus psorosis virus. Plant Pathol. 2010;59:982–90.
Velázquez K, Pina JA, Navarro L, Moreno P, Guerr J. Association of citrus psorosis B symptoms with a sequence variant of the Citrus psorosis virus RNA2. Plant Pathol. 2012;61:448–56.
Vidalakis G, da Graça JV, Dixon WN, Ferrin D, Kesinger M, Krueger RR, Lee RF, Melzer MJ, Olive J, Polek M, Sieburth PJ, Williams LL, Wright GC. Citrus Quarantine Sanitary and Certification Programs in the USA. Citrograph. 2010;3:26–35.
Voinnet O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001;17(8):499–500.
Zanek MC, Peña E, Reyes CA, Figueroa J, Stein B, Grau O, Garcia ML. Detection of Citrus psorosis virus in the northwestern citrus production area of Argentina by using an improved TAS-ELISA. J Virol Methods. 2006;137:245–51.
Zanek MC, Reyes CA, Cervera M. Genetic transformation of sweet orange with the coat protein gene of Citrus psorosis virus and evaluation of resistance against the virus. Plant Cell Rep. 2008;27:57–66.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achachi, A., Ait Barka, E. & Ibriz, M. Recent advances in Citrus psorosis virus . VirusDis. 25, 261–276 (2014). https://doi.org/10.1007/s13337-014-0199-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-014-0199-7