Abstract
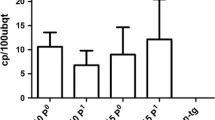
Damage caused by Citrus psorosis virus (CPsV) could be avoided obtaining resistant cultivars by breeding, but the sensitivity of most citrus hosts to CPsV is unknown. To find potential sources of resistance we inoculated 63 cultivars and hybrids of Citrus and related genera [Citrus (36), Microcitrus (5), Fortunella (6), Eremocitrus (1), Pleiospermium (1), Atalantia (2), Severinia (1), Clausena (1), Swinglea (1), Afraegle (1), Poncirus (1) and hybrids (7)] with the CPsV isolate PB-143 and monitored symptoms and CPsV infection by ELISA. Microcitrus inodora and Fortunella hindsii were symptomless but gave high ELISA values, suggesting tolerance to CPsV, whereas Citrus depresa, Clausena excavata, Cleopatra mandarin, sour orange, Carrizo citrange and CPB4475 citrumelo were ELISA negative, suggesting resistance. Further examination of CPsV infection by reverse transcription quantitative real time PCR (RT-qPCR) in Cleopatra mandarin, Poncirus trifoliata and Carrizo citrange seedlings inoculated with CPsV isolates P-121, PB-102 and PB-143, revealed different infection rates and CPsV accumulation, depending on the isolate and citrus genotype. Bud propagation of these genotypes on CPsV-inoculated sweet orange plants incited a bud union disorder that hindered flushing and scion growth, suggesting that the high viral load in the rootstock induced a hypersensitive-like reaction in the partially resistant scion.



Similar content being viewed by others
References
Alioto, D., Gangemi, M., Deaglio, S., Sposato, S., Noris, E., Luisoni, E., & Milne, R. G. (1999). Improved detection of citrus psorosis virus using polyclonal and monoclonal antibodies. Plant Pathology, 48, 735–741.
Ancillo, G., Gadea, J., Forment, J., Guerri, J., & Navarro, L. (2007). Class prediction of closely related plant varieties using gene expression profiling. Journal of Experimental Botany, 58, 1927–1933.
Arregui, J. M., Ballester, J. F., Pina, J. A., & Navarro, L. (1982). Influencia del sustrato y de la fertilización en el crecimiento de plantas de lima Mejicana (Citrus aurantifolia (Christm.) Swing) cultivadas en invernadero. Anales INIA Serie Agrícola, 19, 61–82.
Barthe, G. A., Ceccardi, T. L., Manjunath, K. L., & Derrick, K. S. (1998). Citrus psorosis virus: nucleotide sequencing of the CP gene and detection by hybridization and RT-PCR. Journal of General Virology, 79, 1531–1537.
Beñatena, H. N., & Portillo, M. M. (1984). Natural spread of psorosis in sweet orange seedlings. In S. M. Garnsey, L. W. Timmer, & J. A. Dodds (Eds.), Proceedings of the 9th conference of the international organization of citrus virologists (pp. 159–164). Riverside: IOCV.
Comellas, M. (2009). Estudio de la interacción entre naranjo amargo y el virus de la tristeza de los cítricos. PhD Thesis, Universidad Politécnica de Valencia, Spain.
Dawson, T. E., & Mooney, P. A. (2000). Evidence for trifoliate resistance breaking isolates of Citrus tristeza virus in New Zealand. In J. V. da Graça, R. F. Lee, & R. K. Yokomi (Eds.), Proceedings of the 14th conference of the international organization of citrus virologists (pp. 69–76). Riverside: IOCV.
Sánchez de la Torre, E., Riva, O., Zandomeni, R., Grau, O., García, M. L. (1998). The top component of citrus ringspot virus contains two ssRNAs, the smaller encodes the coat protein. Molecular Plant Pathology Online [http://194.247.68.33/mppol/1998/ 1019sanchez/].
Derrick, K. S., Brlansky, R. H., da Graça, J. V., Lee, R. F., Timmer, L. W., & Nguyen, T. K. (1988). Partial characterization of a virus associated with citrus ringspot. Phytopathology, 78, 1298–1301.
Duran-Vila, N., & Moreno, P. (2000). Enfermedades de los cítricos. Madrid: Ediciones Mundi-Prensa.
Fawcett, H. S., & Bitancourt, A. A. (1943). Comparative symptomatology of psorosis varieties on citrus in California. Phytopathology, 33, 837–864.
Fawcett, H. S., & Klotz, L. J. (1938). Types and symptoms of psorosis and psorosis-like diseases of citrus. Phytopathology, 28, 670.
Galipienso, L., Navarro, L., Ballester-Olmos, J. F., Pina, J. A., Moreno, P., & Guerri, J. (2000). Host range and symptomatology of a graft-transmissible pathogen causing bud union crease of citrus on trifoliate rootstocks. Plant Pathology, 49, 308–314.
Garnsey, S. M., Su, H. J., & Tsai, M. C. (1996). Differential susceptibility of pummelo and Swingle citrumelo to isolates of Citrus tristeza virus. In J. V. da Graça, P. Moreno, & R. K. Yokomi (Eds.), Proceedings of the 13th conference of the international organization of citrus virologists (pp. 138–146). Riverside: IOCV.
Jones, J. D. G., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323–329.
King, A. M. K., Adams, M. J., Carstens, E. B., & Lefkowitz, E. J. (2012). Virus taxonomy: classification and nomenclature of viruses. Ninth report of the international committee on taxonomy of viruses. San Diego: Elsevier-Academic Press.
Klotz, L. J., & Fawcett, H. S. (1941). Color handbook of citrus diseases. Berkeley: University of California Press.
Loconsole, G., Saponari, M., & Savino, V. (2010). Development of real-time PCR based assays for simultaneous and improved detection of citrus viruses. EuropeanJournal of Plant Pathology, 128, 251–259.
Luna, G. R., Peña, E. J., Borniego, M. B., Heinlein, M., & García, M. L. (2013). Ophioviruses CPsV and MiLBVV movement protein is encoded in RNA 2 and interacts with the coat protein. Virology, 441, 152–161.
Maccheroni, W., Alegria, M. C., Greggio, C. C., Piazza, J. P., Kamla, R. F., Zacharias, P. R. A., Bar-Joseph, M., Kitajima, E. W., Assumpção, L. C., Camarotte, G., Cardozo, J., Casagrande, E. C., Ferrari, F., Franco, S. F., Giachetto, P. F., Girasol, A., Jordão Júnior, H., Silva, V. H. A., Souza, L. C. A., Aguilar-Vildoso, C. I., Zanca, A. S., Arruda, P., Kitajima, J. P., Reinach, F. C., Ferro, J. A., & Da Silva, A. C. R. (2005). Identification and genomic characterization of a new virus (Tymoviridae Family) associated with Citrus Sudden Death disease. Journal of Virology, 79, 3028–3037.
Mandadi, K. K., & Scholthof, K.-B. G. (2013). Plant immune response against viruses: how does a virus cause disease? Plant Cell, 25, 1489–1505.
Martín, S., Alioto, D., Milne, R. G., Guerri, J., & Moreno, P. (2002). Detection of Citrus psorosis virus in field trees by direct tissue blot immunoasay in comparison with ELISA, symptomatology, biological indexing and cross-protection tests. Plant Pathology, 51, 134–141.
Martín, S., Alioto, D., Milne, R. G., Garnsey, S. M., García, M. L., Grau, O., Guerri, J., & Moreno, P. (2004). Detection of Citrus psorosis virus by ELISA, molecular hybridization, RT-PCR and immunosorbent electron microscopy and its association with citrus psorosis disease. European Journal of Plant Pathology, 110, 747–757.
Martín, S., López, C., García, M. L., Naum-Onganía, G., Grau, O., Flores, R., Moreno, P., & Guerri, J. (2005). The complete nucleotide sequence of a Spanish isolate of Citrus psorosis virus: comparative analysis with other ophioviruses. Archives of Virology, 150, 167–176.
Milne, R. G., García, M. L., & Grau, O. (2000). Genus ophiovirus. Type species Citrus psorosis virus (CPsV). In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, & R. B. Wickner (Eds.), virustaxonomy, 7th report of the international committee on taxonomy of viruses (pp. 627–631). San Diego: Academic.
Miyakawa, T., & Matsui, C. (1988). The association of tatter leaf virus with budunion crease of trees on trifoliate orange rootstock. In L. W. Timmer, S. M. Garnsey, & L. Navarro (Eds.), Proceedings of the 10th conference of the international organization of citrus virologists (pp. 360–364). Riverside: IOCV.
Moreno, P., Ambrós, S., Albiach-Martí, M. R., Guerri, J., & Peña, L. (2008). Citrus tristeza virus a pathogen the changed the course of the citrus industry. Molecular Plant Pathology, 9, 251–268.
Naum-Onganía, G., Gago-Zachert, S., Peña, E., Grau, O., & García, M. L. (2003). Citrus psorosis virus RNA 1 is of negative polarity and potentially encodes in its complementary strand a 24K protein of unknown function and 280K putative RNA dependent RNA polymerase. Virus Research, 96, 49–61.
Navarro, L. (1993). Citrus sanitation, quarantine and certification programs. In P. Moreno, J. V. da Graça, & L. W. Timmer (Eds.), Proceedings of the 12th conference of the international organization of citrus virologists (pp. 383–391). Riverside: IOCV.
Navas-Castillo, J., & Moreno, P. (1993). Biological diversity of citrus ringspot isolates in Spain. Plant Pathology, 42, 347–357.
Reyes, C. A., De Francesco, A., Peña, E. J., Costa, N., Plata, M. I., Sendin, L., Castagnaro, A. P., & García, M. L. (2011). Resistance to Citrus psorosis virus in transgenic sweet orange plants is triggered by coat protein–RNA silencing. Journal of Biotechnology, 151, 151–158.
Roistacher, C. N. (1991). Graft-transmissible diseases of citrus. Handbook for detection and diagnosis. Rome: FAO.
Roistacher, C. N. (1993). Psorosis—a review. In P. Moreno, J. V. da Graça, & L. W. Timmer (Eds.), Proceedings of the 12th conference of the international organization of citrus virologists (pp. 139–154). Riverside: IOCV.
Román, M. P., Cambra, M., Juárez, J., Moreno, P., Duran-Vila, N., Tanaka, F. A. O., Alves, E.:., Kitajima, E. W., Yamamoto, P. T., Bassanezi, R. B., Teixeira, D. C., Jesus Junior, W. C., Ayres, A. J., Gimenes-Fernandes, N., Rabenstein, F., Girotto, L. F., & Bové, J. M. (2004). Sudden death of citrus in Brazil: A graft-transmissible bud union disease. Plant Disease, 88, 453–467.
Ruiz-Ruiz, S., Ambrós, S., Vives, M. C., Navarro, L., Moreno, P., & Guerri, J. (2009). Detection and quantitation of Citrus leaf blotch virus by TaqMan real-time RT-PCR. Journal of Virological Methods, 160, 57–62.
Sánchez de la Torre, E., López, C., Grau, O., & García, M. L. (2002). RNA2 of Citrus psorosis virus is of negative polarity and has a single open reading frame in its complementary strand. Journal of General Virology, 83, 1777–1781.
Timmer, L. W., & Garnsey, S. M. (1980). Natural spread of citrus ringspot virus in Texas and its association with psorosis-like diseases in Florida and Texas. In E. C. Calavan, S. M. Garnsey, & L. W. Timmer (Eds.), Proceedings of the 8th conference of the international organization of citrus virologists (pp. 167–173). Riverside: IOCV.
Velázquez, K., Renovell, A., Comellas, M., Serra, P., García, M. L., Pina, J. A., Navarro, L., Moreno, P., & Gerri, J. (2010). Effect of temperature on RNA silencing of a negative stranded RNA plant virus: Citrus psorosis virus. Plant Pathology, 59, 982–990.
Vives, M. C., Velázquez, K., Pina, J. A., Moreno, P., Guerri, J., & Navarro, L. (2013). Identification of a new enamovirus associated with citrus vein enation disease by deep sequencing of small RNAs. Phytopathology, 103, 1077–1086.
Wallace, J. M., & Drake, R. J. (1962). Tatter leaf, a previously undescribed virus effect on citrus. Plant Disease Reporter, 46, 211–214.
Acknowledgments
We thank Maria Boil for technical assistance in the laboratory.
Conflict of interest
This work was supported by grant AGL2012-32429 co-financed by FEDER and the Ministerio de Economia y competitividad.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 169 kb)
Rights and permissions
About this article
Cite this article
Velázquez, K., Alba, L., Zarza, O. et al. The response of different genotypes of citrus and relatives to Citrus psorosis virus inoculation. Eur J Plant Pathol 144, 73–81 (2016). https://doi.org/10.1007/s10658-015-0751-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0751-3