Abstract
Background and Objective
It remains unclear whether sepsis in patients with malignancy interferes with the predictive performance of the dose-estimation formulas. The quick sequential organ failure assessment (qSOFA) score can help identify patients with poor outcomes because of sepsis-associated organ damage. Vancomycin, an important antibiotic, treats systemic infections (sepsis) caused by methicillin-resistant Staphylococcus aureus. We aimed to clarify whether including the qSOFA score in a standard population pharmacokinetic (PopPK) assessment may improve the predictive performance of vancomycin doses in patients with malignancy.
Methods
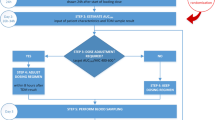
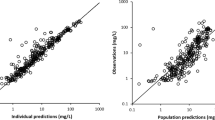
This was a retrospective, observational study. Serum vancomycin concentration–time datasets were obtained from the therapeutic drug monitoring records of St. Luke’s International Hospital (Tokyo, Japan) from January 2011 to August 2016. Clinical and laboratory data of the relevant patients were retrieved from electronic health records. PopPK analysis was performed using the NONMEM program, which includes creatinine clearance (CLCr), blood neutrophil counts, qSOFA scores, and type of malignancy as covariates. We examined the validity of the final PopPK model using bootstrapping, goodness-of-fit plots, and prediction-corrected visual predictive checks.
Results
Six hundred and eight blood samples were obtained from 325 patients. In the final PopPK model, the CLCr and qSOFA scores were selected as covariates of systemic vancomycin clearance (p < 0.05): the population mean value was 2.8 (L/h). Regardless of the CLCr, a qSOFA score of greater than 1 was associated with an approximately 10% reduction in vancomycin clearance.
Conclusions
qSOFA scores might be an additional covariate to CLCr for estimating vancomycin concentrations with a PopPK model in patients with malignancy.


Similar content being viewed by others
Change history
18 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13318-024-00890-8
References
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835–64. https://doi.org/10.1093/ajhp/zxaa036.
Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, et al. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit. 1998;20:139–48. https://doi.org/10.1097/00007691-199804000-00003.
Yamamoto M, Kuzuya T, Baba H, Yamada K, Nabeshima T. Population pharmacokinetic analysis of vancomycin in patients with Gram-positive infections and the influence of infectious disease type. J Clin Pharm Ther. 2009;34:473–83. https://doi.org/10.1111/j.1365-2710.2008.01016.x.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. https://doi.org/10.1159/000180580.
Aljutayli A, Marsot A, Nekka F. An update on population pharmacokinetic analyses of vancomycin Part I. In adults. Clin Pharmacokinet. 2020;59:671–98. https://doi.org/10.1007/s40262-020-00866-2.
Sadoh S, Tsuji Y, Tsukamoto K, Tsukamoto K. Correlation of pharmacokinetic parameters with serum vancomycin concentration in elderly patients with malignancies. Yakugaku Zasshi. 2010;130:69–73. https://doi.org/10.1248/yakushi.130.69.
Shimamoto Y, Fukuda T, Tanaka K, Komori K, Sadamitsu D. Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med. 2013;39:1247–52. https://doi.org/10.1007/s00134-013-2909-9.
Irikuchi J, Imai T, Yoshida Y, Orii T. Influence of systemic inflammatory response syndrome on the pharmacokinetics of vancomycin. Yakugaku Zasshi. 2015;135:745–51. https://doi.org/10.1248/yakushi.14-00214.
Chuma M, Makishima M, Imai T, Tochikura N, Sakaue T, Kikuchi N, et al. Duration of systemic inflammatory response syndrome influences serum vancomycin concentration in patients with sepsis. Clin Ther. 2016;38:2598–609. https://doi.org/10.1016/j.clinthera.2016.10.009.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–74. https://doi.org/10.1001/jama.2016.0288.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. https://doi.org/10.1053/j.ajkd.2008.12.034.
Kellum JA, Lameire NH, Aspelin P. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–38.
He J, Yang ZT, Qian X, Zhao B, Mao EQ, Chen EZ, et al. A higher dose of vancomycin is needed in critically ill patients with augmented renal clearance. Transl Androl Urol. 2020;9:2166–71. https://doi.org/10.21037/tau-20-1048.
Suzuki M, Kasai H, Sako K. Population pharmacokinetic analysis of vancomycin in adult cancer patients with neutropenia effect. The Jpn J Ther Drug Monit. 2019;36:96–104.
Rushing TA, Ambrose PJ. Clinical application and evaluation of vancomycin dosing in adults. Journal of Pharmacy Technology. 2016;17:33–8. https://doi.org/10.1177/875512250101700201.
Kim AJ, Lee JY, Choi SA, Shin WG. Comparison of the pharmacokinetics of vancomycin in neurosurgical and non-neurosurgical patients. Int J Antimicrob Agents. 2016;48:381–7. https://doi.org/10.1016/j.ijantimicag.2016.06.022.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51. https://doi.org/10.1208/s12248-011-9255-z.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247. https://doi.org/10.1007/s00134-021-06506-y.
Katip W, Jaruratanasirikul S, Pattharachayakul S, Wongpoowarak W, Jitsurong A, Lucksiri A. The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect Drug Resist. 2016;9:253–60. https://doi.org/10.2147/IDR.S121513.
Goncalves-Pereira J, Martins A, Povoa P. Pharmacokinetics of gentamicin in critically ill patients: pilot study evaluating the first dose. Clin Microbiol Infect. 2010;16:1258–63. https://doi.org/10.1111/j.1469-0691.2009.03074.x.
Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med. 2017;45:1443–9. https://doi.org/10.1097/CCM.0000000000002538.
Nakayama H, Suzuki M, Kato T, Echizen H. Vancomycin pharmacokinetics in patients with advanced cancer near end of life. Eur J Drug Metab Pharmacokinet. 2019;44:837–43. https://doi.org/10.1007/s13318-019-00564-w.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39:420–3. https://doi.org/10.1016/j.ijantimicag.2011.12.011.
Teramachi H, Matsushita R, Tsuji A. Influence of malignancy on the pharmacokinetics of vancomycin hydrochloride in Japanese MRSA patients after dosage adjustment with the Bayesian method. Jpn J Chemother. 2005;53:357–63.
Aoyama S, Teramachi H, Miyazato T. Investigation for factors affecting clearance of vancomycin. The Jpn J Ther Drug Monit. 2013;30:1–5.
Alqahtani S, Almatrafi A, Bin Aydan N, Alqahtani M, Alzamil F, Alsultan A, et al. Optimization of vancomycin dosing regimen in cancer patients using pharmacokinetic/pharmacodynamic modeling. Pharmacotherapy. 2020;40:1192–200. https://doi.org/10.1002/phar.2475.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no external funding.
Conflict of Interest
The authors declare no conflicts of interest directly relevant to the content of the present study.
Availability of Data and Material
All software used in the present study is commercially available and the necessary information was given in the text.
Ethics Approval
The study protocol was approved by the Research Ethics Committee of St. Luke’s International Hospital (approval number 16-074).
Consent to Participate
Written informed consent from participating patients was waived because all blood samples for TDM were obtained for therapeutic purposes as well as other medical procedures under the comprehensive agreement of patients. The opt-out statement for the present study was disclosed to the hospital’s public statement board.
Consent to Publication
Not applicable.
Code Availability
The original code of the population pharmacokinetic model may be available upon a reasonable request from the corresponding author.
Author’s Contributions
YT, MT, and FW designed the research. YT collected the data. YT and MT performed the data analysis and the pharmacokinetic analysis. YT wrote the draft manuscript. YT, MT, FW, KG, and HE substantially contributed to revising the manuscript drafts. All authors approved the final version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuda, Y., Takahashi, M., Watanabe, F. et al. Population Pharmacokinetic Analysis of Vancomycin in Patients with Solid or Hematological Malignancy in Relation to the Quick Sequential Organ Failure Assessment Scores. Eur J Drug Metab Pharmacokinet 48, 647–655 (2023). https://doi.org/10.1007/s13318-023-00850-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00850-8