Abstract
Background and objective
There is no consensus on the optimal vancomycin dose to achieve pharmacokinetic/pharmacodynamic (PK/PD) target in patients with hematologic cancer or in hematopoietic stem cell transplant (HSCT) recipients. A 24-h area under the concentration-time curve (AUC) >400 mg*h/L must be achieved early for successful treatment of severe methicillin-resistant Staphylococcus aureus (MRSA) infections. Current nomograms derived from general population data are not sufficiently accurate to allow AUC-based model-informed precision dosing. The objective of this study was to characterize vancomycin PK in patients with hematologic cancer or in HSCT recipients and to develop a model-informed dosing tool based on PK/PD target requirements.
Methods
Pooled retrospective and prospective vancomycin serum concentrations were analyzed using NONMEM® to evaluate the performance of previously published population PK (popPK) models built from hematologic cancer datasets and to develop a novel Bayesian PK model. Patients’ characteristics and clinical data were tested as potential covariates. The popPK model was validated internally and externally. Predictions of vancomycin concentrations for different dosing regimens were made using Monte-Carlo simulations, and a nomogram strategy was proposed according to selected probability of target attainment (PTA).
Results
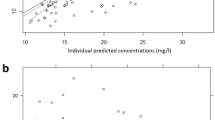
The predictive performance of the published popPK models was found to be suboptimal for our population. A novel popPK model was developed using 240 vancomycin concentrations (60 patients). A two-compartment structural model with an additive error model best described the data. Ideal body weight and estimated glomerular filtration rate (eGFR) [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)] were selected as covariates for volume of distribution (V) and clearance (CL). Bootstrapping confirmed the stability and precision of the popPK parameters. The volume of distribution was V1 = 46.8 L and V2 = 56.1 L, while CL = 5.63 L/h. External validation using 107 vancomycin concentrations (24 patients) demonstrated the predictivity of the model. A nomogram was developed to reach minimally PTA >50% for 400 < AUC < 600 mg*h/L.
Conclusion
To our knowledge, this study provides the first model-informed AUC-based strategy in North American hematologic cancer patients with or without HSCT. The resulting nomogram generated provides a simplified approach to improving the accuracy of initial vancomycin dosing in this population.




Similar content being viewed by others
References
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-93.
Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am. 2011;25(1):101–16.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prevention and Treatment of Cancer-Related Infections. National Comprehensive Cancer Network. 2022; version 3.
Martin JH, Norris R, Barras M, Roberts J, Morris R, Doogue M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin Biochem Rev. 2010;31(1):21–4.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Lodise TP, Scheetz M, Carreno JJ, Chambers H, Fowler V, Holland TL. Associations between vancomycin exposure and acute kidney injury within the recommended area under the curve therapeutic exposure range among patients with methicillin-resistant staphylococcus aureus bloodstream infections. Open Forum Infect Dis. 2022;9(2): ofab651.
Lodise TP, Drusano G. Vancomycin area under the curve-guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant staphylococcus aureus infections: putting the safety of our patients first. Clin Infect Dis. 2021;72(9):1497–501.
Wicha SG, Märtson A-G, Nielsen EI, Koch BCP, Friberg LE, Alffenaar J-W, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–41.
He N, Dong F, Liu W, Zhai S. A systematic review of vancomycin dosing in patients with hematologic malignancies or neutropenia. Infect Drug Resist. 2020;13:1807–21.
Ghehi MT, Rezaee S, Hayatshahi A, Hadjibabaie M, Gholami K, Javadi M, et al. Vancomycin pharmacokinetic parameters in patients undergoing hematopoietic stem cell transplantation (HSCT). Int J Hematol Oncol Stem Cell Res. 2013;7(4):1–9.
Arjangpour S, Sadeghi K, Solduzian M, Mousavi SA. Vancomycin pharmacokinetic parameters in patients undergoing hematopoietic stem cell transplantation. J Oncol Pharm Pract. 2022;28(1):101–8.
Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988;32(6):848–52.
Nakashima T, Ohno T, Koido K, Hashimoto H, Terakado H. Accuracy of predicting the vancomycin concentration in Japanese cancer patients by the Sawchuk-Zaske method or Bayesian method. J Oncol Pharm Pract. 2020;26(3):543–8.
Buelga D, Del Mar Fernandez De Gatta M, Herrera EV, Dominguez-Gil A, Garcia MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother. 2005;49(12):4934–41.
Fernández de Gatta Mdel M, Santos Buelga D, Sánchez Navarro A, Dominguez-Gil A, García MJ. Vancomycin dosage optimization in patients with malignant haematological disease by pharmacokinetic/pharmacodynamic analysis. Clin Pharmacokinet. 2009;48(4):273–80.
Bury D, Ter Heine R, van de Garde EMW, Nijziel MR, Grouls RJ, Deenen MJ. The effect of neutropenia on the clinical pharmacokinetics of vancomycin in adults. Eur J Clin Pharmacol. 2019;75(7):921–8.
Haeseker MB, Croes S, Neef C, Bruggeman CA, Stolk LM, Verbon A. Vancomycin dosing in neutropenic patients. PLoS ONE. 2014;9(11): e112008.
Choi MH, Choe YH, Lee SG, Jeong SH, Kim JH. Neutropenia is independently associated with sub-therapeutic serum concentration of vancomycin. Clin Chim Acta. 2017;465:106–11.
Rosner MH, Perazella MA. Acute kidney injury in the patient with cancer. Kidney Res Clin Pract. 2019;38(3):295–308.
Heus A, Uster DW, Grootaert V, Vermeulen N, Somers A, In’t Veld DH, et al. Model-informed precision dosing of vancomycin via continuous infusion: a clinical fit-for-purpose evaluation of published PK models. Int J Antimicrob Agents. 2022;59(5): 106579.
Belabbas T, Yamada T, Egashira N, Hirota T, Suetsugu K, Mori Y, et al. Population pharmacokinetic model and dosing optimization of vancomycin in hematologic malignancies with neutropenia and augmented renal clearance. J Infect Chemother. 2023;29(4):391–400.
Okada A, Kariya M, Irie K, Okada Y, Hiramoto N, Hashimoto H, et al. Population pharmacokinetics of vancomycin in patients undergoing allogeneic hematopoietic stem-cell transplantation. J Clin Pharmacol. 2018;58(9):1140–9.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98.
Mandell B, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed; 2005.
McCarron MM, Devine BJ. Clinical pharmacy: case studies: case number 25 gentamicin therapy. Drug Intell Clin Pharm. 1974;8(11):650–5.
Nyman U, Leander P, Liss P, Sterner G, Brismar T. Absolute and relative GFR and contrast medium dose/GFR ratio: cornerstones when predicting the risk of acute kidney injury. Eur Radiol. 2023. https://doi.org/10.1007/s00330-023-09962-w. (Epub 4 Aug 2023).
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–66.
Haeseker M, Croes S, Neef C, Bruggeman C, Stolk L, Verbon A. Evaluation of vancomycin prediction methods based on estimated creatinine clearance or trough levels. Ther Drug Monit. 2016;38(1):120–6.
Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO Jr. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575–80.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9.
Milone G, Bellofiore C, Leotta S, Milone GA, Cupri A, Duminuco A, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: a review based on physiopathology. J Clin Med. 2022;11(3):623.
Rondón G, Saliba RM, Chen J, Ledesma C, Alousi AM, Oran B, et al. Impact of fluid overload as new toxicity category on hematopoietic stem cell transplantation outcomes. Biol Blood Marrow Transplant. 2017;23(12):2166–71.
Aljutayli A, Marsot A, Nekka F. An update on population pharmacokinetic analyses of vancomycin, Part I. In adults. Clin Pharmacokinet. 2020;59(6):671–98.
Yamamoto M, Kuzuya T, Baba H, Yamada K, Nabeshima T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J Clin Pharm Ther. 2009;34(4):473–83.
Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–54.
Landry DWBH. Approach to the patient with renal disease. In: Godlman L, Schafer AI, editors. Goldman-Cecil Medicine. 26th ed. Philadelphia: Elsevier; 2020.
Hosten AO. BUN and Creatinine. In: Walker HK, Hall WD, Hurst JW (eds). Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths Copyright © 1990, Butterworth Publishers, a division of Reed Publishing; 1990.
Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433–7.
Okada N, Chuma M, Azuma M, Nakamura S, Miki H, Hamano H, et al. Effect of serum concentration and concomitant drugs on vancomycin-induced acute kidney injury in haematologic patients: a single-centre retrospective study. Eur J Clin Pharmacol. 2019;75(12):1695–704.
Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Stewart JJ, Jorgensen SC, Dresser L, Lau TT, Gin A, Thirion DJ, et al. A Canadian perspective on the revised 2020 ASHP-IDSA-PIDS-SIDP guidelines for vancomycin AUC-based therapeutic drug monitoring for serious MRSA infections. J Assoc Med Microbiol Infect Dis Can. 2021;6(1):3–9.
Zhao W, Zhang D, Fakhoury M, Fahd M, Duquesne F, Storme T, et al. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob Agents Chemother. 2014;58(6):3191–9.
Jarkowski IA, Forrest A, Sweeney RP, Tan W, Segal BH, Almyroudis N, et al. Characterization of vancomycin pharmacokinetics in the adult acute myeloid leukemia population. J Oncol Pharm Pract. 2012;18(1):91–6.
Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51(1):1–13.
Xu XS, Yuan M, Karlsson MO, Dunne A, Nandy P, Vermeulen A. Shrinkage in nonlinear mixed-effects population models: quantification, influencing factors, and impact. AAPS J. 2012;14(4):927–36.
Acknowledgements
The authors thank Michel Savoie for his precious feedback at every step of this project and for reviewing the manuscript. The authors also thank Asma Ghorbaniyanamirkhiz and Danielle Fany Ngontié Tcheudjio for participating in protocol writing, subject recruitment, and data collection; Sandrine Sinju-Caron for participating in data collection; and Bogdan Andrei Panait for his participation in the recruitment of the prospective subjects. Amélie Marsot acknowledges support from the Fonds de Recherche du Québec-Santé (FRQS) Research Scholars-Junior 1 (Young Researcher Establishment) Career Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Amélie Marsot received financial support from the Fonds de Recherche du Québec-Santé (FRQS) Research Scholars-Junior 1 (Young Researcher Establishment) Career Scholarship.
Conflicts of Interest
Jessica Le Blanc, Denis Projean, Sandra Savignac, Sophie Léveillé, Marie-Pier Ducas, Annie Brisebois-Boyer, and Amélie Marsot declare they have no competing interests associated with this publication.
Ethics Approval
This clinical trial was approved by the local human research and ethics committee (REB) [CIUSSS de l’Est-de-l’Île-de-Montréal - 2021-2482] and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent to Participate and for Publication
All participants in the prospective cohort provided written consent to participate. Authorization from the local REB was obtained to collect data retrospectively from chart review.
Data Availability
Individual patient data from Maisonneuve-Rosemont Hospital is not publicly accessible.
Code Availability
The code is available from the corresponding author on reasonable request.
Authors’ Contributions
Conception and design: All authors. PK model development and related computational analysis: AM. Statistical analysis: JLB, DP, AM. Data interpretation: All authors. Writing – original draft preparation: DP, JLB, AM, AB. Writing – review and editing: All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Blanc, J., Projean, D., Savignac, S. et al. Toward Model-Based Informed Precision Dosing of Vancomycin in Hematologic Cancer Patients: A First Step. Clin Pharmacokinet 63, 183–196 (2024). https://doi.org/10.1007/s40262-023-01329-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01329-0