Abstract
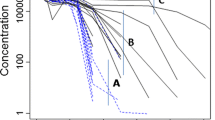
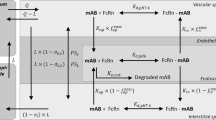
Population pharmacokinetic (PopPK) model parameter estimation and predictive performance depend on the data adequacy for model building. PopPK models of therapeutic monoclonal antibodies (mAbs) may not be well supported by commonly used sparse sampling in late-stage development because of the slow absorption (days) and long half-life (weeks) of mAbs, affecting accuracy of predicted exposure metrics which are often used to support drug development. A case study was presented for a representative mAb to compare the predictive performance of two established PopPK models from their respective data. Differences in datasets for model building (including sample size, sampling schedule and route of administration), model structure and parameters, and key derived exposure metrics were compared, and the resulting differences in model prediction were elaborated. With the majority of the data used for developing models being trough concentration (Ctrough) data, both models projected similar Ctrough and area under the concentration-time curve (AUC) but different peak concentrations (Cmax) at steady state following the same subcutaneous dose regimen. Our case study supports the importance of appropriate sampling schemes for PopPK model development and exposure metric estimation. We recommend collecting proper random pharmacokinetic samples, in addition to troughs, to allow adequate characterization of PopPK models for mAbs. Selecting the informative model and relevant pharmacokinetic metrics could be critical in driving drug development decision-making, especially in simulation-based exposure matching to inform doses in special populations such as pediatrics.

Similar content being viewed by others
References
Williams P, Ette E. Pharmacometrics: impacting drug development and pharmacotherapy. In: Ette E, Williams P, editors. Pharmacometrics: the science of quantitative pharmacology. Hoboken: John Wiley & Sons Inc.; 2007. p. 1–21.
Xu Y, Kimko H. Pharmacometrics: a quantitative decision-making tool in drug development. In: Marchenko OV, Katenka NV, editors. Quantitative methods in pharmaceutical research and development. Berlin: Springer Press; 2020. p. 71–104.
US Food and Drug Administration (USFDA). Population Pharmacokinetics. Food and Drug Administration-Guidance for Industry. 2019. https://www.fda.gov/media/128793/download.pdf. Accessed 28 Dec 2020.
US Food and Drug Administration (USFDA). General clinical pharmacology considerations for pediatric studies for drugs and biological products—Guidance for Industry. December 2014. https://www.fda.gov/media/90358/download. Accessed 28 Dec 2020.
Grist B, Zheng S, Xu Y. Therapeutic antibody development—remington chapter. In: Adeboye A, editor. Remington. Cambridge: Academic Press; 2021. p. 437–62.
Mentre F, Mallet A, Baccar D. Optimal design in random-effects regression models. Biometrika. 1997;84:429–42.
Li J, Li D, Chen Y. Assessing predictive performance of pharmacokinetic models by limited sampling—a case study of therapeutic monoclonal antibody. Clin Pharmacol Ther. 2021;109:S42 (Abstract PII-050).
Dumont C, Lestini G, Le Nagard H, et al. PFIM 40, an extended R program for design evaluation and optimization in nonlinear mixed-effect models. Comput Methods Programs Biomed. 2018;156:217–29.
Foracchia M, Hooker A, Vicini P, Ruggeri A. POPED, a software for optimal experiment design in population kinetics. Comput Methods Programs Biomed. 2004;74:29–46.
Stockmann C, Barrett JS, Roberts JK, Sherwin C. Use of modeling and simulation in the design and conduct of pediatric clinical trials and the optimization of individualized dosing regimens. CPT Pharmacometrics Syst Pharmacol. 2015;4:630–40.
Lee JY, Garnett CE, Gobburu JV, et al. Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin Pharmacokinet. 2011;50:627–35.
Hu C, Zhou H, Sharma A. Landmark and longitudinal exposure-response analyses in drug development. J Pharmacokinet Pharmacodyn. 2017;44:503–7.
Hsu CH, Xu X, Wang J, Zhang L, Liu C, Wang Y. Utilization of adult data in designing pediatric pharmacokinetic studies: How much are historical adult data worth? J Clin Pharmacol. 2019;59:989–96.
Mould DR, Meibohm B. Drug development of therapeutic monoclonal antibodies. BioDrugs. 2016;30:275–93.
Zhao L, Ren Th, Wang D. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33:1339–47.
Zhao L, Shang EY, Sahajwalla CG. Application of pharmacokinetics-pharmacodynamics/clinical response modeling and simulation for biologics drug development. J Pharm Sci. 2012;101:4367–82.
Sun A, Benet LZ. Late-stage failures of monoclonal antibody drugs: a retrospective case study analysis. Pharmacology. 2020;105:145–63.
Ogasawara K, Breder CD, Lin DH, Alexander GC. Exposure- and dose-response analyses in dose selection and labeling of FDA-approved biologics. Clin Ther. 2018;40:95-102.e2.
Acknowledgments
The authors gratefully acknowledge Honghui Zhou and Zhenhua Xu (Clinical Pharmacology and Pharmacometrics, Janssen Research & Development, LLC, Spring House, PA, USA) for their expert review of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this work.
Conflicts of Interest
Yang Chen and Chuanpu Hu are employees of Janssen Research & Development, LLC, Pharmaceutical Companies of Johnson & Johnson, and may own stock and/or stock options in the company. Joshua Li and Derry Li declared no conflicts of interest for this work. The opinions expressed in this article are those of the authors and not necessarily those of the company that employs them.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson, Inc. is available at https://www.janssen.com/clinical-trials/transparency.
Code Availability
Code is available upon request.
Author Contributions
Yang Chen and Chuanpu Hu conceived and planned the analysis. Joshua Li and Derry Li conducted model simulations, data analysis and production of tables and figures. All authors contributed to interpretation of results and writing of the manuscript. Joshua Li and Derry Li are high school students and participated in this study with permissions from their schools.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, J., Li, D. et al. Pharmacokinetic Modeling and Predictive Performance: Practical Considerations for Therapeutic Monoclonal Antibodies. Eur J Drug Metab Pharmacokinet 46, 595–600 (2021). https://doi.org/10.1007/s13318-021-00707-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00707-y