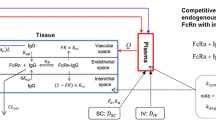
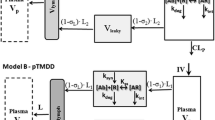
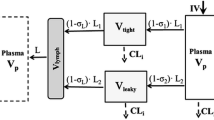
Abstract
Physiologically based pharmacokinetic (PBPK) models can be used to leverage physiological and in vitro data to predict monoclonal antibody (mAb) concentrations in serum and tissues. However, it is currently not known how consistent predictions of mAb disposition are across PBPK modelling platforms. In this work PBPK simulations of IgG, adalimumab and infliximab were compared between three platforms (Simcyp, PK-Sim, and GastroPlus). Accuracy of predicted serum and tissue concentrations was assessed using observed data collected from the literature. Physiological and mAb related input parameters were also compared and sensitivity analyses were carried out to evaluate model behavior when input values were altered. Differences in serum kinetics of IgG between platforms were minimal for a dose of 1 mg/kg, but became more noticeable at higher dosages (> 100 mg/kg) and when reference (healthy) physiological input values were altered. Predicted serum concentrations of both adalimumab and infliximab were comparable across platforms, but were noticeably higher than observed values. Tissue concentrations differed remarkably between the platforms, both for total- and interstitial fluid (ISF) concentrations. The accuracy of total tissue concentrations was within a three-fold of observed values for all tissues, except for brain tissue concentrations, which were overpredicted. Predictions of tissue ISF concentrations were less accurate and were best captured by GastroPlus. Overall, these simulations show that the different PBPK platforms generally predict similar mAb serum concentrations, but variable tissue concentrations. Caution is therefore warranted when PBPK models are used to simulate effect site tissue concentrations of mAbs without data to verify the predictions.





Similar content being viewed by others
References
Tang Y, Li X, Cao Y (2021) Which factors matter the most? Revisiting and dissecting antibody therapeutic doses. Drug Discovery Today 26:1980–1990. https://doi.org/10.1016/j.drudis.2021.04.022
Shah DK, Betts AM (2013) Antibody biodistribution coefficients. MAbs 5:297–305. https://doi.org/10.4161/mabs.23684
Glassman PM, Abuqayyas L, Balthasar JP (2015) Assessments of antibody biodistribution. J Clin Pharmacol 55:S29–S38. https://doi.org/10.1002/jcph.365
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709. https://doi.org/10.1007/s10928-007-9065-1
Lobo ED, Hansen RJ, Balthasar JP (2004) Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 93:2645–2668. https://doi.org/10.1002/jps.20178
Jadhav SB, Khaowroongrueng V, Derendorf H (2016) Microdialysis of large molecules. J Pharm Sci 105:3233–3242. https://doi.org/10.1016/j.xphs.2016.08.016
Dragatin C, Polus F, Bodenlenz M et al (2016) Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp Dermatol 25:157–159. https://doi.org/10.1111/exd.12863
Liu S, Shah DK (2022) Mathematical models to characterize the absorption, distribution, metabolism, and excretion of protein therapeutics. Drug Metab Dispos 50:867–878. https://doi.org/10.1124/dmd.121.000460
Covell DG, Barbet J, Holton OD et al (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Res 46:3969–3978
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54:1517–1528
Ferl GZ, Wu AM, DiStefano JJ (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652. https://doi.org/10.1007/s10439-005-7410-3
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86. https://doi.org/10.1007/s10928-011-9232-2
Gill KL, Gardner I, Li L, Jamei M (2016) A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J 18:156–170. https://doi.org/10.1208/s12248-015-9819-4
Niederalt C, Kuepfer L, Solodenko J et al (2018) A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J Pharmacokinet Pharmacodyn 45:235–257. https://doi.org/10.1007/s10928-017-9559-4
Zhou H, Bolger M, Lukacova V (2015) Application of PBPK modeling to predict monoclonal antibody disposition after intravenous and subcutaneous administration in rats and humans [ABSTRACT]. In: AAPS. Orlando, FL
den Broeder A, van de Putte L, Rau R et al (2002) A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol 29:2288–2298
Weisman MH, Moreland LW, Furst DE et al (2003) Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 25:1700–1721. https://doi.org/10.1016/S0149-2918(03)80164-9
Palaparthy R, Udata C, Hua SY et al (2018) A randomized study comparing the pharmacokinetics of the potential biosimilar PF-06438179/GP1111 with Remicade® (infliximab) in healthy subjects (REFLECTIONS B537–01). Expert Rev Clin Immunol 14:329–336. https://doi.org/10.1080/1744666X.2018.1446829
Park W, Lee SJ, Yun J, Yoo DH (2015) Comparison of the pharmacokinetics and safety of three formulations of infliximab (CT-P13, EU-approved reference infliximab and the US-licensed reference infliximab) in healthy subjects: a randomized, double-blind, three-arm, parallel-group, single-dose, Phase I study. Expert Rev Clin Immunol 11:25–31. https://doi.org/10.1586/1744666X.2015.1090311
Kavanaugh A, St Clair EW, McCune WJ et al (2000) Chimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 27:841–850
EMEA (2004) Humira EPAR - scientific disussion
FDA (1999) Remicade (infliximab) for IV injection - supplement approval
Suzuki T, Ishii-Watabe A, Tada M et al (2010) Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 184:1968–1976. https://doi.org/10.4049/jimmunol.0903296
Kaymakcalan Z, Sakorafas P, Bose S et al (2009) Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol 131:308–316. https://doi.org/10.1016/j.clim.2009.01.002
Garg A, Balthasar JP (2009) Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J 11:553–557. https://doi.org/10.1208/s12248-009-9129-9
Denney WS, Duvvuri S, Buckeridge C (2015) Simple, automatic noncompartmental analysis: the PKNCA R package. J Pharmacokinet Pharmacodyn 42(11–107):S65. https://doi.org/10.1007/s10928-015-9432-2
Rohatgi A (2021) Webplotdigitizer: Version 4.5
Rafidi H, Rajan S, Urban K et al (2022) Effect of molecular size on interstitial pharmacokinetics and tissue catabolism of antibodies. mAbs 14:2085535. https://doi.org/10.1080/19420862.2022.2085535
Jadhav SB, Khaowroongrueng V, Fueth M et al (2017) Tissue distribution of a therapeutic monoclonal antibody determined by large pore microdialysis. J Pharm Sci 106:2853–2859. https://doi.org/10.1016/j.xphs.2017.03.033
Chang HP, Cheung YK, Shah DK (2021) Whole-body pharmacokinetics and physiologically based pharmacokinetic model for monomethyl Auristatin E (MMAE). J Clin Med 10:1332. https://doi.org/10.3390/jcm10061332
Jin F, Tayab ZR, Balthasar JP (2006) Pharmacokinetic and pharmacodynamic effects of high-dose monoclonal antibody therapy in a rat model of immune thrombocytopenia. AAPS J 7:E895-902. https://doi.org/10.1208/aapsj070487
Viala M, Vinches M, Alexandre M et al (2018) Strategies for clinical development of monoclonal antibodies beyond first-in-human trials: tested doses and rationale for dose selection. Br J Cancer 118:679–697. https://doi.org/10.1038/bjc.2017.473
Mahmood I, Tegenge MA, Golding B (2020) Considerations for optimizing dosing of immunoglobulins based on pharmacokinetic evidence. Antibodies 9:24. https://doi.org/10.3390/antib9020024
Ryman JT, Meibohm B (2017) pharmacokinetics of monoclonal antibodies. CPT: pharmacometrics & systems. Pharmacology 6:576–588. https://doi.org/10.1002/psp4.12224
Wu S, Le Prieult F, Phipps CJ et al (2022) PBPK model for antibody disposition in mouse brain: validation using large-pore microdialysis data. J Pharmacokinet Pharmacodyn 49:579–592. https://doi.org/10.1007/s10928-022-09823-x
Mandikian D, Figueroa I, Oldendorp A et al (2018) Tissue physiology of cynomolgus monkeys: cross-species comparison and implications for translational pharmacology. AAPS J 20:107. https://doi.org/10.1208/s12248-018-0264-z
Abdiche YN, Yeung YA, Chaparro-Riggers J et al (2015) The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. MAbs 7:331–343. https://doi.org/10.1080/19420862.2015.1008353
Dickinson BL, Badizadegan K, Wu Z et al (1999) Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest 104:903–911. https://doi.org/10.1172/JCI6968
McCarthy KM, Yoong Y, Simister NE (2000) Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci 113:1277–1285. https://doi.org/10.1242/jcs.113.7.1277
Funding
This work received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: AV, EG, P-JDS; Methodology: AV, EG, P-JDS; Formal analysis and investigation: P-JDS; Writing—original draft preparation: P-JDS; Writing—review and editing: AV, EG, P-JDS; Supervision: AV.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Sutter, PJ., Gasthuys, E. & Vermeulen, A. Comparison of monoclonal antibody disposition predictions using different physiologically based pharmacokinetic modelling platforms. J Pharmacokinet Pharmacodyn (2023). https://doi.org/10.1007/s10928-023-09894-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10928-023-09894-4