Abstract
Sansevieria trifasciata showing symptoms of leaf blight were observed in several states of Malaysia. Based on morphology and DNA sequences of the Internal Transcribed Spacer (ITS) region, nine isolates of Neoscytalidium dimidiatum were identified. Phylogenetic analysis grouped the isolates of N. dimidiatum from S. trifasciata with the epitype of Neoscytalidium dimidiatum (CBS499.66) from mango. Pathogenicity test showed that all isolates of N. dimidiatum caused leaf blight on S. trifasciata and fulfilled Koch’s postulates.
Similar content being viewed by others
Sansevieria trifasciata, commonly known as mother-in-law’s tongue, is a popular ornamental plant that can be found throughout Malaysia. Besides its attractive dark and light green variegation, S. trifasciata is tolerant to a wide variety of habitats. Sansevieria trifasciata were reported to be infected by Colletotrichum sansevieriae (Nakamura et al. 2006) and Chaetomella sp. (Li et al. 2013) associated with anthracnose and leaf spot, respectively. In 2015, another disease symptom, leaf blight was observed on S. trifasciata in several states of Malaysia. Scytalidium-like fungal isolates were recovered during preliminary fungal isolation from the samples.
During September 2015 and March 2017, diseased S. trifasciata with symptoms of leaf blight were collected from Penang and three other Malaysian states, namely Perak, Negeri Sembilan and Sarawak. Symptomatic samples were cut into approximately 1.5 cm2 fragments and surface sterilised in 70% ethanol (C2H5OH) solution and 1% sodium hypochlorite (NaOCl) solution for 3 min each. Then, the samples were rinsed thrice in sterile distilled water for 1 min each before being plated on potato dextrose agar (PDA) and incubated at 25 ± 3 °C for 3 to 5 days. Pure cultures were obtained by transferring a single conidium from the colony grown from surface sterilised samples onto fresh PDA and incubated as described previously. A mycelial plug 6 mm in diameter taken from a 7-days-old culture was transferred onto a PDA plate. The growth rate was recorded by measuring the colony diameter daily until the mycelia fully covered the plate. The isolates were cultured on 2% water agar (WA) overlaid with sterilised horsetail twigs (Casuarina equisetifolia) and carnation leaves, then were incubated as described previously to induce pycnidia formation. Measurements of 50 randomly selected conidia from pycnidia and arthrospores were taken.
A total of nine Scytalidium-like fungal isolates were obtained from diseased S. trifasciata showing leaf blight symptoms in four different states (Penang, Perak, Negeri Sembilan and Sarawak). Morphologically, all the isolates were identified as N. dimidiatum (Crous et al. 2006; Chuang et al. 2012; Masratul Hawa et al. 2013). Colonies on PDA were greyish white, gradually became greenish dark grey, dense with arthrospores and flat aerial mycelia, with dark pigmentation (Fig. 1a and b). Mycelial growth rate was 4.3 ± 1.0 cm/day on PDA. Sizes of arthric conidia ranged from 6.2 × 3.3 μm to 10.2 × 5.5 μm, 0 to 1 septate, hyaline to brown, and circular, oval or cylindrical with round to truncate ends (Fig. 1c). Conidia exuded in milky white cirrhus from pycnidia were one-celled, aseptate, oblong and 11.4 ± 0.7 μm × 4.9 ± 0.3 μm (Fig. 1d). All nine isolates of N. dimidiatum were deposited in Culture Collection Unit, Department of Plant Pathology, School of Biological Sciences, Universiti Sains Malaysia. Both sterile carnation leaves and horsetail twigs were used to induce the formation of pycnidia (Fig. 1e and f). However, pycnidia on carnation leaves tended to be covered by mycelia and arthric conidia, thus the horsetail twig was more suitable for examination of single-cell conidium from the conidiomata.
Morphological characteristics of N. dimidiatum isolate, (a) colony of N. dimidiatum on PDA, (b) pigmentation of N. dimidiatum on PDA, (c) arthric conidia, (d) conidia from pycnidia, (e) pycnidia and mycelia on carnation leaf, (f) pycnidia with conidia exuded from cirrhus on horsetail twig. Bars: c = 20 μm, d = 20 μm, e = 1000 μm, f = 200 μm
Invisorb Spin Plant Mini Kit (Stratec, Germany) was used to extract the DNA of the isolates. The extraction was done according to the manufacturer’s instructions. Amplification of the ITS region was conducted using primers ITS1 and ITS4 (White et al. 1990).
PCRs were prepared in 50 μl containing 8 μl 5× GoTaq® Green Buffer (Promega, USA), 8 μl MgCl2 (25 mM) (Promega, USA), 1 μl dNTP Mix (10 mM) (Promega, USA), 0.8 μM of each primer (ITS1 and ITS4), 0.3 μl Taq polymerase (Promega, USA) and 0.6 μl genomic DNA. PCRs were run in MyCycler™ Thermal Cycler (Bio-Rad, Hercules, CA, USA) that was programmed for an initial denaturation at 95 °C for 5 min, followed by 35 cycles (each 30 s at 95 °C, 30 s at 54 °C and 1 min at 72 °C) prior to a final extension of 5 min at 72 °C. PCR products were sent to a service provider for DNA sequencing.
Sequences were aligned and analysed using MEGA7 (Kumar et al. 2016). The resulting sequences were deposited in GenBank. Based on BLAST searches, eight sequences (Accession No. MF580792-MF580799) and one sequence (KX401435) showed 100% (579/579) and 98% (572/583) identity to the epitype of Neoscytalidium dimidiatum (CBS499.66), respectively.
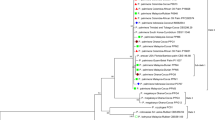
Phylogenetic relationships of the isolates were analysed using MEGA7. The ITS sequence for the type specimen of N. dimidiatum was incorporated for analysis using maximum likelihood (ML) method after the best model of nucleotide substitution was selected (Tamura 3-parameter). Bootstrap values were determined from 1000 bootstrap replicates. Gaps and missing data were eliminated. Isolates used in the phylogenetic study, including the type specimen and outgroups are listed in Table 1. Of the 590 characters in the multiple alignments, 522 were conserved sites, 58 were variable sites and 31 were parsimony informative sites. ML analysis based on ITS sequences indicated that all isolates of N. dimidiatum in the present study were grouped in the same clade with N. dimidiatum CBS499.66, supported by bootstrap value of 71% (Fig. 2).
Pathogenicity of all isolates obtained was assessed on potted S. trifasciata using conidial suspension and mycelial plug. Fungal conidial suspensions (1 × 106 conidia/ml) and mycelial plugs (6 mm in diameter) were prepared from 7-days-old cultures on PDA plates. A total of 0.1 ml of conidial suspension was injected into the leaves whereas mycelial plugs were placed with their upper surface facing downward on wounds made by a sterile needle. Sterile distilled water and PDA plugs without mycelia were used as controls. All control and inoculated plants were maintained in a plant house, School of Biological Sciences, Universiti Sains Malaysia at 25 to 31 °C for 2 weeks. Fungal isolates were then re-isolated from the symptomatic inoculated plants and re-identified. The experiment was repeated twice and three replicates were prepared for each isolate.
Inoculation of both methods on potted plants resulted in lesions with darkened centres around the wounded sites that expanded as browning to chlorosis of the tissues (Fig. 3a and b). Under humid conditions, mycelia with arthric conidia were observed on the lesions. No symptoms were observed on the controls. The pathogen was consistently re-isolated from the inoculated leaves of S. trifasciata, and morphological examination showed that it was N. dimidiatum.
Neoscytalidium dimidiatum is an opportunistic pathogen of various hosts causing different types of diseases (Padin et al. 2005). This pathogen was reported to cause stem canker (Masratul Hawa et al. 2013; Sanahuja et al. 2016) and brown rot of dragon fruit (Chuang et al. 2012), canker of English walnut (Chen et al. 2013), leaf blight of white spider lily (Nurul Nadiah et al. 2017), dieback of lesser yam (Lin et al. 2017), dieback, stem-end rot and canker of mango (Sakalidis et al. 2011; Marques et al. 2013), wood canker of grapevine (Rolshausen et al. 2013), shoot blight, canker, and gummosis of citrus (Polizzi et al. 2009), human toenail infection (da Silva et al. 2016) and rhinosinusitis (Bakhshizadeh et al. 2014). The occurrence of N. dimidiatum on S. trifasciata contributes to the knowledge of the host range of this plant pathogenic fungus. Etiological information of N. dimidiatum will help to develop strategies and efforts to control this important pathogen that can pose a threat to plants and humans. To our knowledge, this is the first report of N. dimidiatum causing leaf blight on S. trifasciata in Malaysia and worldwide.
References
Bakhshizadeh M, Hashemian HR, Najafzadeh MJ, Dolatabadi S, Zarrinfar H (2014) First report of rhinosinusitis caused by Neoscytalidium dimidiatum in Iran. J Med Microbiol 63:1017–1019
Chen SF, Fichtner E, Morgan DP, Michailides TJ (2013) First report of Lasiodiplodia citricola and Neoscytalidium dimidiatum causing death of graft union of English walnut in California. Plant Dis 97(7):993
Chuang MF, Ni HF, Yang HR, Shu HL, Lai SY, Jiang YL (2012) First report of stem canker disease of pitaya (Hylocereus undatus and H. polyrhizus) caused by Neoscytalidium dimidiatum in Taiwan. Plant Dis 96(6):906
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WF, Philips AJ, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
da Silva RT, Guimaraes DA, Camargo ZP, Rodrigues AM, Maceira JP, Bernardes-Engemann AR, Orofino-Costa R (2016) Cutaneous murine model of infection caused by Neoscytalidium dimidiatum: a preliminary study of an emerging human pathogen. Med Mycol 54:890–898
Huang SK, Tangthirasunun N, Phillips AJL, Dai DQ, Wanasinghe DN, Wen TC, Bahkali AH, Hyde KD, Kang JC (2016) Morphology and phylogeny of Neoscytalidium orchidacearum sp. nov. (Botryosphaeriaceae). Mycobiology 44(2):79–84
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Li YL, Zhou Z, Lu W, Ye JR (2013) First report of a Chaetomella sp. causing a leaf spot on Sansevieria trifasciata in China. Plant Dis 97(7):992
Lin CH, Chen YX, Liu WB, WQ W, Miao WG, Zheng FC (2017) First report of Dioscorea esculenta dieback caused by Neoscytalidium dimidiatum in China. Plant Dis 101(7):1320
Marques MW, Lima NB, de Morais MA Jr, Michereff SJ, Phillips AJL, Camara MPS (2013) Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum species associated with mango in Brazil. Fungal Divers 61:195–208
Masratul Hawa M, Salleh B, Latiffah Z (2013) Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J Phytopathol 161(11–12):841–849
Nakamura M, Ohzono M, Iwai H, Arai K (2006) Anthracnose of Sansevieria trifasciata caused by Colletotrichum sansevieriae sp. nov. J Gen Plant Pathol 72:253–256
Nurul Nadiah MA, Mohamed Nor NMI, Latiffah Z, Masratul Hawa M (2017) First report of leaf blight on white spider lily caused by Neoscytalidium dimidiatum in Malaysia. New Dis Rep 35:16
Padin C, Fernández-Zeppenfeldt G, Yegres F, Richard-Yegres N (2005) Scytalidium dimidiatum: an opportunistic fungus for both man and Mangifera indica trees in Venezuela. Rev Iberoam Micol 22(3):172–173
Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GE, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100(6):851–866
Phillips AJ, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76(1):51–167
Polizzi G, Aiello D, Vitale A, Giuffrida F, Groenewald Z, Crous PW (2009) First report of shoot blight, canker, and gummosis caused by Neoscytalidium dimidiatum on citrus in Italy. Plant Dis 93:1215
Rolshausen PE, Akgul DS, Perez R, Eskalen A, Gispert C (2013) First report of wood canker caused by Neoscytalidium dimidiatum on grapevine in California. Plant Dis 97(11):1511
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GES, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. Eur J Plant Pathol 130:379–391
Sanahuja G, Lopez P, Palmateer AJ (2016) First report of Neoscytalidium dimidiatum causing stem and fruit canker of Hylocereus undatus in Florida. Plant Dis 100(7):1499
Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2005) Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 97(1):99–110
van Niekerk JM, Crous PW, Groenewald JZ, Fourie PH, Halleen F (2004) DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96(4):781–798
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press INc., New York), pp 315–322
Acknowledgements
This work was supported by Short Term Grant (304/PBIOLOGI/6313226) from Universiti Sains Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kee, Y.J., Suhaimi, N.N., Zakaria, L. et al. Characterisation of Neoscytalidium dimidiatum causing leaf blight on Sansevieria trifasciata in Malaysia. Australasian Plant Dis. Notes 12, 60 (2017). https://doi.org/10.1007/s13314-017-0284-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13314-017-0284-z