Abstract
Ambrosia beetles have co-evolved symbiotic relationships with an array of fungal partners. Mutualistic fungal partners are often highly successful in vertical transmission between beetle generations. These persisting relationships can alter beetle behaviour, resulting in the opportunity to occupy new ecological niches and to spread geographically. In Australia, ambrosia beetles are not currently considered a significant pest in commercial Pinus plantations, where the bark beetle Ips grandicollis is known as the primary invader of stressed trees. However, in 2019, ambrosia beetles Xyleborus perforans and X. bispinatus, co-occurring with I. grandicollis, were found to have colonised a large proportion of drought-stressed trees in commercial Pinus plantations in north-east New South Wales. In this study, X. perforans (the most prevalent of two Xyleborus spp.) was collected from infested dead and dying trees in two NSW Pinus plantations. Fungal isolates of suspected Pinus pathogens were recovered from beetle mycangia and exoskeletons as well as ambrosia beetle galleries. Morphological examination and multilocus sequence analysis identified five fungi associated with X. perforans: Fusarium parceramosum, Fusarium aff. solani, Ophiostoma ips, Raffaelea deltoideospora and Sporothrix pseudoabietina. For Australia, this is the first report of F. parceramosum, as well as the first records of O. ips, R. deltoideospora and S. pseudoabietina being vectored by Xyleborus. Pathogenicity tests were performed on seedlings of three Pinus spp., with O. ips producing significantly longer lesions than the other fungi. This study demonstrates the potential for seemingly harmless ambrosia beetles to vector plant pathogens in Australian forests, providing a mode of disease transmission that should be considered in plantation management and forest biosecurity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
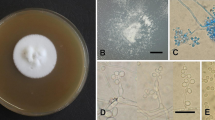
The New South Wales (NSW) forestry sector is a major contributor to the Australian economy and supports a range of social and environmental sustainability benefits (Department of Agriculture, Fisheries and Forestry ABARES 2018). NSW pine plantations (Pinus spp.) span over 306,000 hectares, accounting for 78% of the state’s commercial plantations, and are currently valued at $393 million (Australian Bureau of Agricultural and Research Economics and Sciences 2019). These plantations are often established on land unsuitable for agricultural production, as trees are considered more tolerant to a wide variety of site factors including soil types and rainfall events (Department of Agriculture, Fisheries and Forestry ABARES 2018; Forrest 1973). In some instances, however, trees may become stressed and predisposed to opportunistic insect pests, including ambrosia (Curculionidae: Scolytinae and Platypodinae) and bark (Curculionidae: Scolytinae) beetles (Flechtmann et al. 2001; Lynch et al. 2016; Stone et al. 2010; Wylie et al. 1999; Yousuf et al. 2014). During annual forest health surveys in north-east NSW in February 2019, unusual symptoms were observed in severely drought-stressed Pinus taeda, P. elliottii and P. elliottii x caribaea plantations, including dark-red staining and resin bleeding (Fig. 1a, b). Closer inspection revealed colonisation by the bark beetle Ips grandicollis Eichhoff and by the ambrosia beetles Xyleborus perforans Wollaston and X. bispinatus Eichhoff (Fig. 1c, d); all three of which are established exotics in Australia (Stone et al. 2010). Forest health surveillance of these plantations, performed annually over the past 25 years (Carnegie et al. 2008), has shown I. grandicollis to be the most common invader of stressed pine trees (Elliott et al. 1998; Forestry Corporation of New South Wales 2022; Wylie et al. 1999; Yousuf et al. 2014). In Australia, X. perforans and X. bispinatus are known to occupy conifers in north-east NSW (Stone et al. 2010) but are generally restricted to colonising felled trees (Wylie et al. 1999) or dying and dead standing trees as was observed in Queensland in 1992 (Elliott et al. 1998). The colonisation by Xyleborus spp. on stressed standing trees has not previously been reported in NSW.
External signs of Xyleborus colonisation on drought-stressed Pinus trees in north-east NSW: a dark-red stained trees, b often with red resin exudate, c white frass and d dark orange frass. Dark orange frass most commonly indicates presence of I. grandicollis, whereas white to light brown frass piles indicate Xyleborus species. This is because frass colour is relative to tree material being consumed by beetles, phloem or xylem tissue for Ips spp. and Xyleborus spp., respectively
Ambrosia beetles spend most of their life cycle residing in galleries that penetrate xylem wood tissue (Musvuugwa et al. 2015) and have co-evolved morphological adaptations that aid the transmission of fungal associates (Harrington 2005; Popa et al. 2012). Some of these fungal partners have been shown to play a key nutritional role in the ambrosia beetle lifecycle and are a main food source during larval development or provide nutrients essential for beetle reproduction (Batra 1966; Klepzig and Six 2004; Musvuugwa et al. 2015). Despite this, many of the ambrosia beetle-fungus relationships are understudied, with growing evidence of symbioses where the fungal partner may also assist the beetle in overcoming tree defences resulting in novel pathosystems in invaded ranges (Joseph and Keyhani 2021). A key adaptation in ambrosia beetle-fungus relationships is the beetle’s development of specialised fungal transport organs, called mycangia (Kostovcik et al. 2015; Six 2003) which have evolved independently across several ambrosia beetle phylogenetic lineages (Joseph and Keyhani 2021; Li et al. 2018b; Li et al. 2019). The location of mycangia also differs between these independently evolved clades of ambrosia beetles, existing, for example, as exoskeletal pores (e.g., on the ventral posterior prothorax), internal sacs (described as mandibular or oral pockets (i.e., openings near or within beetle’s oral cavity), or mesonatally (i.e., located between the pronotum and elytron) (Joseph and Keyhani 2021; Kostovcik et al. 2015; Li et al. 2018a).
The genus Xyleborus stores mutualistic, nutritional fungi selectively in their dual preoral pocket mycangia (Batra 1963; Cruz et al. 2019; Francke-Grossman 1967; Menocal et al. 2023; Saucedo et al. 2017). Related genera, such as Xylosandrus Reitter and Euwallacea Hopkins (Curculionidae: Xylosandrus and Scolytinae, respectively), also demonstrate consistent associations of fungi in mycangia (Joseph and Keyhani 2021; Thu et al. 2021). Xyleborus spp., however, have more promiscuous fungal associations in comparison to these other genera (e.g., Xylosandrus spp.), hosting a greater variety of fungi in high abundance (Kostovcik et al. 2015). In some cases, several nutritional fungi have been found occupying Xyleborus spp. mycangia at the same time (Saucedo-Carabez et al. 2018). Alternatively, the propagation of non-nutritional fungi can also occur passively via external transmission on the beetle’s exoskeleton, or internally through their guts (Hulcr and Stelinski 2017), however, the putative symbiotic roles of other fungal groups found from Xyleborus spp. remain unknown (Kostovcik et al. 2015). The composition of a beetle’s fungal community, whether passively or actively transmitted, can change through the acquisition of fungi from the environment, especially if in an invaded range (Morales-Rodriguez et al. 2021; Rassati et al. 2019). With many of these associations, the transmission of fungi by beetles is considered to be harmless, potentially resulting in the spread of nutritional fungal symbionts between tree hosts, nevertheless, plant pathogens are also capable of being transmitted by ambrosia beetles to healthy trees, resulting in tree diseases with significant economic and environmental impacts (Alamouti et al. 2009; Aoki et al. 2019; Harrington 2005; Klepzig and Six 2004; Lynch et al. 2016; Webber 1990).
Some of the most common fungal associates of ambrosia beetles are found to reside within the Ophiostomatales Benny and Kimbr., Microascales Luttr. ex Benny and Kimbr., and the Ambrosia Fusarium Clade (AFC) of the Hypocreales Lindau (Alamouti et al. 2009; Aoki et al. 2019; Harrington 2005; Klepzig and Six 2004; Lynch et al. 2016; Musvuugwa et al. 2015). This group of ambrosial fungi also includes several important tree pathogens (Musvuugwa et al. 2015) that are vectored by beetles both in naïve natural and invaded host ranges (Lynch et al. 2016). Some examples of important tree pathogens vectored by ambrosia beetles include Harringtonia lauricola (= Raffaelea lauricola) (T.C. Harr., Fraedrich and Aghayeva) Z.W. de Beer and M. Procter vectored by Xyleborus glabratus Eichhoff which causes laurel wilt on Lauraceae Juss (including avocados) throughout the USA (Harrington et al. 2008), Japanese oak die-back caused by Dryadomyces quercivorus (= Raffaelea quercivora) (Kubono and Shin. Ito) M. Procter and Z.W. de Beer which is vectored by the oak ambrosia beetle (Platypus quercivorus Murayama (Kubono and Ito 2002)), and Fusarium dieback caused by Fusarium euwallaceae Freeman which is vectored by the polyphagous shot-hole borer (Euwallacea fornicatus Eichhoff) (Salman et al. 2019). In Australia, there is limited knowledge of ambrosia beetle-fungus associations, leaving an array of understudied organisms, interactions, and modes of pathogen transmission that may be significant in forest health management (Musvuugwa et al. 2015).
Globally, Xyleborus spp. are well-documented to establish promiscuous fungal associations in invaded ranges (Kostovcik et al. 2015; Lynch et al. 2016; Morales-Rodriguez et al. 2021; Rassati et al. 2019; Saucedo-Carabez et al. 2018). These associates are, however, complex, varied and dependent on the geographical location of the beetle and its tree host (Joseph and Keyhani 2021). In Australia, the associations between ambrosia beetles and fungi remain unknown. As such, a closer examination of the diversity of fungi and their relationships with ambrosia beetles in Australia is needed, particularly assessing the potential of Xyleborus spp. to vector plant pathogenic fungi. The objectives of this study were to (1) identify potential pathogenic fungi vectored by ambrosia beetles that were found colonising P. elliottii and P. caribaea in northern NSW plantations, and (2) to compare the pathogenicity of these associated fungi on three Pinus species commonly grown in NSW commercial pine plantations.
Materials and methods
Site location and sample collection
Beetle samples were collected from two commercial plantations near Whiporie in north-east NSW: Barragunda Plantation (29° 0′ 57.8'' S, 152° 58′ 13.5'' E) and Banyabba State Forest (29° 20′ 38.7'' S, 153° 0′ 30.2'' E). Collections from Barragunda Plantation were made in February 2019 and April 2020, while Banyabba State Forest was sampled in June 2020. At each sampling site, P. elliottii x P. caribaea trees infested with Xyleborus spp. (and co-colonised by I. grandicollis) were felled and sectioned to collect the beetles from their galleries and the inner bark layer. At least 50 Xyleborus beetles were collected at each site and stored together in 50 mL sterile collection tubes at 3ºC until being processed within 21 days of collection. Beetles of the same species collected from the same tree were stored together. A separate collection tube was used if a new species or tree was sampled. Similarly, at least 5 billets or sections of billets from felled trees were collected and stored together in enclosed plastic tubs at 3ºC until being processed within 28 days of collection. A subset of beetles from each location were sent to the NSW Department of Primary Industries Insect Diagnostic Unit for morphological identification, with the remainder being used for fungal isolations. Both X. perforans and X. bispinatus were recovered from infested trees, however, X. perforans was found in substantially greater numbers (as has previously been observed in trapping studies (Stone et al. 2010)), and therefore this species was focused on for the remainder of the study.
Fungal isolations
Fungal isolations were performed on three substrates per sample: (1) Xyleborus perforans exoskeleton, (2) mycangia and (3) ambrosial galleries sectioned from infested wood. To sample from beetle exoskeletons, fungal spores were dislodged and recovered by vortexing 73 beetles individually in a 1.5 mL Eppendorf tube containing 500 μL of 0.01% sterilised Tween20™ (Biochemicals) for 3 min (Alamouti et al. 2006). A 50 μL aliquot of the spore suspension was then spread onto quarter strength potato dextrose agar (Sigma-Aldrich) amended with 0.01% streptomycin (Astral Scientific) (¼ PDA-strep) to limit the growth of bacteria or yeasts (Biedermann et al. 2009).
To select for mycangial fungi (i.e. fungi located within the dual preoral pocket mycangia) we followed the methods of Kostovcik et al. (2015). A total of 144 X. perforans beetles across the two sites were surface sterilised aseptically by soaking them in 500 μL of 80% v/v ethanol (Chem Supply Pty Ltd) for 10 s and rinsing three times in sterile Milli-Q water. Individual beetles were then decapitated aseptically, and the head was macerated in a 1.5 mL micro-centrifuge tube with 200 μL sterile Milli-Q water using a sterile rod. After briefly vortexing (10 s), the resulting suspensions were spread onto ¼ PDA-strep plates, with the remaining tissue plated separately.
To access ambrosial galleries, bark was removed, and pine billets split with an axe to access unexposed internal galleries that were located in the centre of the billet. Surface tissues, such as bark, were removed during this process and kept separate from the exposed galleries to avoid cross-contamination between ambrosial galleries and I. grandicollis galleries located within the bark layer. A total of 111 wood sections from Xyleborus spp. gallery systems were aseptically cut from the billet using a sterile hammer and chisel and surface sterilised using 80% v/v ethanol, rinsed in sterile Milli-Q water three times, placed on ¼ PDA-strep plates and incubated. Sections taken were no larger than 5 mm × 5 mm and taken from the leading edge of symptomatic tissue or a gallery cross-section.
All ¼ PDA-strep plates were incubated at room temperature (18 – 23 ºC) and checked for spore germination twice daily for the first 3 days, and then daily for the subsequent 11 days using an Olympus SZX7 stereomicroscope. Germinating single spores or individual mycelial tips were subcultured onto unamended ¼ PDA and incubated as above to produce axenic cultures. Morphological examinations were performed using an Olympus BX51 microscope with Nomarski differential interference contrast (DIC) optics. Fungal isolates were grouped based on micro- and macro-morphology and colony characteristics, with representative strains lodged at the NSW Plant Pathology Herbarium (Table 1).
DNA extraction, PCR amplification and sequencing
Fungal genomic DNA was extracted from axenic cultures using the REDExtract-N-AmpTM Plant PCR-Kit (Sigma-Aldrich) according to the manufacturer’s instructions. Commonly sequenced phylogenetic loci for each genus were amplified according to protocols described in the literature (refer to Supplementary file 1). The PCR reactions employed MyTaq DNA polymerase (Bioline), with reaction composition made up according to the manufacturer’s instructions in a Bio-rad S1000 PCR machine using the following cycling conditions; 1 cycle of 60 s at 95 °C and 35 cycles of 15 s at 95 °C, 15 s at 55 °C (internal transcriber spacer (ITS) region, beta tubulin (BT), nuclear large subunit ribosomal DNA (LSU)) or 51 °C (calmodulin (CAL), translation elongation factor 1 α (TEF1a)) and 10 s at 72 °C, followed by 1 cycle of 5 min at 72 °C. Amplified products were visualised by gel electrophoresis (1% w/v agarose, amended with thiazole orange 15 mg/mL in DMSO; Sigma-Aldrich) and assessed using a Nanodrop 1000 Spectrophotometer (Thermo Scientific, Australia) before purification using the Isolate II PCR and Gel Kit (Bioline). Amplified products were sequenced at the Australian Genome Research Facility, Sydney. Sequences generated in this study were deposited in the NCBI GenBank database (Table 1).
Phylogenetic analyses
Sequences were assembled and quality was assessed in Geneious Prime (Version 2023.0.3) (Biomatters, New Zealand), before forward and reverse reads were assembled to construct consensus sequences (Dreaden et al. 2014). Reference sequences from recent taxonomic studies were included in the phylogenetic analyses of the current study. Sequence alignment was performed using MAFFT (v. 7.490 (Katoh et al. 2002; Katoh and Standley 2013)), with the E-INS-i algorithm and its default parameters, for each locus. Sequence lengths were trimmed to a standardise length for phylogenetic analyses. All aligned sequence datasets for each locus were analysed using RAxML (v. 8) (Stamatakis 2014) to perform maximum likelihood (ML) analysis using the GTR GAMMA I model. Bootstrapping with 1,000 replicates provided confidence support. Fungal species identification was based on concordance at two or more loci (refer to Supplementary file 2).
Pathogenicity testing and analysis
A total of 106 12-month-old Pinus spp. seedlings (26 P. radiata, 40 P. taeda and 39 P. elliottii) with an average stem girth of 6.9 mm (σ = 1.2), 8.1 mm (σ = 1.3) and 8.3 mm (σ = 1.5), respectively, were grown in greenhouse conditions with an average day time temperature of 25oC prior to the comparative pathogenicity test. A 3 mm cork borer was used to excise bark from each seedling stem to create a wound 2 to 3 mm deep (Lynch et al. 2016). Agar plugs taken from the leading edge of 7 to 14-day-old fungal isolates were used to inoculate six replicate seedlings per Pinus spp., with sterile PDA plugs used as a control. Wounds were covered with parafilm which remained for the entire trial period. Seedlings were arranged in a randomised incomplete block design in the glasshouse. After 12 weeks, the presence of lesions and plant responses (stem occlusion) were recorded, and lesion length was measured as the response variable. From each seedling, the recovery of the inoculated isolate from the leading edge of the lesion (if present) was used to confirm Koch’s postulates. If no lesion was present, re-isolation was attempted 2 cm above and below the inoculation site (Lynch et al. 2016). Statistical analysis was conducted in R Studio (version 1.3;1093; R Development Core Team 2016). Lesion length was analysed with a two-way analysis of variance (ANOVA). Individual treatments were compared with Tukey’s multiple comparison HSD test, using the emmeans package (Matusick and Eckhardt 2010).
Results
Fungal isolations from beetles and wood galleries
A total of 333 isolation attempts from beetles and beetle galleries were conducted during this study. Four morphotypes resembling Ophiostomatales (Ophiostoma Syd. and P. Syd., Raffaelea Arx and Hennebert, and Sporothrix Hektoen and C.F. Perkins) and Fusarium Link (Fusarium solani species complex) were recovered from X. perforans beetles and galleries from NSW pine plantations (Table 2). Out of 97 isolates representing these four morphotypes, 46 were recovered from beetle galleries. Fusarium cf. solani (Mart.) Sacc represented 80.4% of isolates recovered from beetle galleries, which was greater than Ophiostoma isolates (15.2%), Raffaelea isolates (2.17%) and Sporothrix isolates (2.17%) (Table 2). Of the remaining 51 isolates recovered, 12 were recovered from beetle mycangia and 39 from beetle exoskeleton (Table 2). From beetle mycangia, F. cf. solani isolates were recovered five times, Ophiostoma isolates were recovered three times and Raffaelea isolates four times (Table 2), whereas no Sporothrix isolates were recovered from beetle mycangia. From beetle exoskeletons, recovery of F. cf. solani isolates represented 69.2% of the total 39 isolates, compared to isolates of Ophiostoma (25.5%) and Sporothrix (10.3%) (Table 2). An abundance of endophytic and saprophytic fungi (e.g., Penicillium spp., Trichoderma spp., Mucor spp., etc.) were also recovered (data not shown).
Identification of fungal isolates
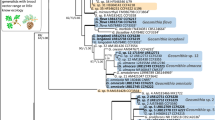
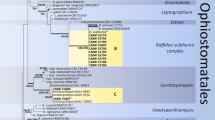
Phylogenetic analyses of ITS regions confirmed the taxonomic placement of each isolate to the genus level, while the additional gene regions of BT, TEF1a, LSU and CAL allowed species identification (refer to Supplementary file 2). Isolates were confirmed to belong in the Fusarium solani species complex (Hypocreales), as well as three genera in the Ophiostomatales, including Ophiostoma, Raffaelea and Sporothrix (Table 1). Taxonomic placement of the representative Fusarium isolates was supported by the TEF1a barcode, with isolates identified to belong in two distinct taxa, namely Fusarium parceramosum (Sand.-Den. and Crous) O'Donnell, Geiser, Kasson and T. Aoki (DAR85310) and a novel species of Fusarium aff. solani (DAR85309, DAR85311).
Representative isolates of the genus Ophiostoma, with near identical ITS sequences, showed greatest homology to Ophiostoma ips (Rumbold) Nannf (DAR85363, DAR85362). Further analyses of LSU obtained for these isolates provided further support and confidence in this identification. Maximum likelihood analysis of ITS and LSU for the Raffaelea representative isolates supported taxonomic placement with greatest homology to Raffaelea deltoideospora (Olchow. and J. Reid) Z.W. de Beer and T.A. Duong (DAR85308). However, only one GenBank reference sequence was comparable for the LSU region (Trollip et al. 2021). A single isolate representing the Sporothrix genus grouped within the S. gossypina (R.W. Davidson) Z.W. de Beer, T.A. Duong and M.J. Wingf. clade. ITS analyses alone provided limited species delineation, however, analysis of CAL confirmed the species as Sporothrix pseudoabietina H.M. Wang, Q. Lu and Zhen Zhang (DAR85304).
Pathogenicity tests
Pathogenicity trials were performed with a representative isolate for the four major taxonomic groups identified in the current study (Figs. 2 and 3). Twelve weeks after wound inoculation isolates of Fusarium aff. solani (DAR85309, DAR85311), S. pseudoabietina (DAR85304) and O. ips (DAR85362, DAR85363) caused significantly longer lesions than the control for all three Pinus spp. (F2, 4 = 41.2, P < 0.001). Seedlings inoculated with O. ips were observed to have significantly longer lesions than all other fungal species (P < 0.001; Fig. 2). Fusarium aff. solani and S. pseudoabietina both caused significantly longer lesions compared to the control seedlings (P < 0.05; Fig. 2). Although R. deltoideospora (DAR85308) produced a lesion in all three Pinus spp. (Fig. 2), it was not significantly longer than the wound lesion on the uninoculated control seedlings (t ratio = -2.63, P > 0.05). For Fusarium aff. solani, S. pseudoabietina and O. ips, there were no significant differences in lesion lengths between the three Pinus spp. tested (P > 0.001).
Upon examination of the inoculated stems, the sapwood in wounded control seedlings were lightly coloured with a light brown outer ring surrounding the inoculation wound site (Fig. 3a). In comparison, dark brown discoloured lesions were often observed near the inoculation site for O. ips (Fig. 3c). Stems inoculated with F. aff. solani (Fig. 3b) and S. pseudoabietina (Fig. 3e) also displayed a brown outer ring, and the lesions were generally observed to be more superficial, closer to the bark layer than O. ips, and not as discoloured (Fig. 3). Lesions present on stems inoculated with R. deltoideospora were observed to be deeper into the sapwood and displayed tissue browning within and around the wound site (Fig. 3d). No differences were observed in lesions and sapwood discolouration between inoculated Pinus spp. seedlings within each fungal treatment (data not shown). Stem occlusion was observed as a response to inoculation in all treatments. However, stem occlusion was observed at higher incidences in all Pinus seedlings of the four fungal treatments compared to control seedlings.
Discussion
The objectives of this study were to identify potential pathogenic fungi vectored by ambrosia beetles that were found to colonise drought-affected P. elliottii and P. caribaea hybrids in northern NSW. Morphological characterisation and phylogenetic analyses identified five fungi associated with X. perforans in two NSW pine plantations: F. parceramosum, F. aff. solani, O. ips, R. deltoideospora and S. pseudoabietina. Pathogenicity testing demonstrated that only O. ips, an already well-known forest pathogen, caused significant lesions and is possibly actively vectored by X. perforans. This may extend the threat O. ips already presents to northern NSW pine plantations through a potential additional beetle vector (X. perforans), alongside its main vector I. grandicollis. The four other fungi are considered to pose a low threat to healthy forests. Our results highlight the potential of promiscuous and phoretic associations in planted forest ecosystems where bark and ambrosia beetles co-inhabit tree hosts of economic importance. This knowledge will inform forest biosecurity and increase our understanding of Australian forest pests and plant pathogen transmission.
Isolates of Fusarium were easily recovered from beetles and galleries, representing the most abundant fungal group collected in the current study (Table 2). This abundance of Fusarium is not surprising as it belongs to a diverse genus known to contain true ambrosia beetle mutualists (Hulcr and Stelinski 2017). These fungal associates of ambrosia beetles are thought to play a vital role in beetle nutrition (Kasson et al. 2013; Kostovcik et al. 2015) or to fill a commensal transient relationship within the beetle’s lifecycle (Joseph and Keyhani 2021). The phylogenetic analysis performed in the current study revealed two closely related taxa which, importantly, cluster outside the Ambrosia Fusarium Clade (AFC) of the Fusarium solani species complex (Aoki et al. 2019; Hulcr and Stelinski 2017; Popa et al. 2012). While further analysis will be required to delimit the Fusarium aff. solani taxon (DAR85309, DAR85311), the TEF1a barcode was able to identify F. parceramosum (DAR85310) – a seemingly ubiquitous fungus which is not considered to be a plant pathogen (Custódio and Pereira 2023; Guarnaccia et al. 2022; Sandoval-Denis et al. 2019). Results of the pathogenicity trial for Fusarium aff. solani taxon in this study, along with previous studies of closely related lineages (Custódio and Pereira 2023; Guarnaccia et al. 2022; Sandoval-Denis et al. 2019), suggest that both these Fusarium taxa are unlikely to cause dieback and death of Pinus hosts. Nonetheless, this serves as Australia’s first official record of F. parceramosum and confirms the presence of cryptic diversity within the Fusarium solani species complex (FSSC) in Australia.
Members of the Ophiostomatales, namely O. ips, S. pseudoabietina and R. deltoideospora, were encountered less abundantly than the Fusarium taxa in our dataset, however, their isolation from X. perforans here is noteworthy. Common associations of ambrosia beetles (Diehl et al. 2023; Malacrinò et al. 2017; Menocal et al. 2023), the Ophiostomatales are possibly the best studied group of bark and ambrosia beetle symbionts with numerous species identified in Pinus plantations across south-eastern Australia (Stone et al. 2010; Trollip et al. 2021; Vaartaja 1967). Ophiostoma ips causes blue stain (sap stain) on Pinus spp. and has been detected across mainland Australia (Hood and Ramsden 1997; Trollip et al. 2021, 2022) and this is both reaffirmed and reflected by the results of the pathogenicity test conducted in the current study. First detected in the 1960s, O. ips is regularly linked to the established exotic bark beetle, I. grandicollis (Hood and Ramsden 1997; Stone et al. 2010; Stone and Simpson 1987; Trollip et al. 2021; Vaartaja 1967) and together, this beetle-fungus association can reduce wood quality and marketability. Here we report X. perforans as a potential new vector for O. ips in Australia, with our results demonstrating the potential of internalised transport of the fungus within the mycangia (active transmission). Similarly, the detection of R. deltoideospora and S. pseudoabietina in this study represents the first records of these fungi in association with X. perforans in Australia. Importantly, neither of these species show the potential to be primary pathogens of Pinus species.
The isolation frequency for each of the four morphotypes observed in this study varied with the tissue type or location on the beetle’s body (Table 2). Similar patterns of isolation have been reported in other ambrosia beetle studies with fungal communities shifting between spatial distinctions from beetle mycangia to exoskeleton to galleries (Aoki et al. 2019). Fusarium spp. and O. ips were both consistently isolated from beetle mycangia, exoskeletons and beetle galleries (Table 2). Raffaelea deltoideospora was only, and infrequently, isolated from beetle mycangia and beetle galleries. Sporothrix pseudoabietina was isolated from the exoskeleton of X. perforans and galleries, but not from beetle mycangia. Previous studies of Raffaelea spp. and Fusarium spp. beetle symbionts (including Xylosandrus spp., Xyleborus spp. and Euwallacea spp.) have identified their putative role as nutritional symbionts based on their spatial distribution on or within their insect vector (Bateman et al. 2016; Cruz et al. 2018; Kasson et al. 2013; Saucedo-Carabez et al. 2018). However, in the present study only low numbers of R. deltoideospora were recovered. It is possible that storage conditions inhibited the recovery of Raffaelea spp. (and other beetle nutritional symbionts) or that they were outcompeted by faster growing fungi (such as the Fusarium species).
Ambrosia beetles, especially in the case of Xyleborus spp., can exhibit high promiscuity with fungal associates in invaded and native ranges (Carrillo et al. 2014; Francke-Grosmann 1967; Kostovcik et al. 2015; Morales-Rodriguez et al. 2021; Rassati et al. 2019). Often, these fungal communities are spatially segregated across an individual beetle (Bateman et al. 2016). As such, the fungal community inadvertently carried by a beetle (passive transmission) can differ from what they carry deliberately via the mycangia (active transmission). The fungal community’s composition may also change due to the acquisition of other fungi from the environment or host, especially in an invaded range (Morales-Rodriguez et al. 2021; Rassati et al. 2019). The recovery of Fusarium spp., O. ips and R. deltoideospora at similar frequencies from beetle mycangia (alongside their recovery from beetle galleries) therefore suggests a status of co-occurring mycangial fungi (Kostovcik et al. 2015; Lynch et al. 2016). Sporothrix pseudoabietina however, was not isolated from the beetle mycangia, suggesting a more phoretic association (Bateman et al. 2016; Chang et al. 2017; Musvuugwa et al. 2015).
While most beetle-fungus relationships are benign, ambrosia beetles can act as the primary mode of transport for plant pathogens into seemingly healthy trees, resulting in the spread of plant disease (Alamouti et al. 2009; Aoki et al. 2019; Gebhardt et al. 2005; Harrington 2005; Klepzig and Six 2004; Lynch et al. 2016; Nkuekam et al. 2011; Webber 1990), such as laurel wilt on Lauraceae vectored by X. glabratus throughout the USA (Harrington et al. 2008). Several Xyleborus species are aggressive pests to healthy trees (Flechtmann et al. 2001; Lynch et al. 2016; Rabaglia 2005) yet the majority have been described as secondary opportunistic pests, as is reported in Australia (Stone et al. 2010; Wylie et al. 1999) and is similar to what was observed in this study with Xyleborus beetles only observed in weak living, dead or dying trees. Additionally, field observations of co-colonisation by X. perforans and I. grandicollis in colonised trees, as well as isolations of F. aff. solani, F. parceramosum, R. deltoideospora and S. pseudoabietina alongside O. ips, makes it difficult to assess whether O. ips is only associated with Xyleborus spp. because of its close proximity to its main vector I. grandicollis. It is possible that O. ips is a mycangial and exoskeleton contaminant or serves another unknown evolutionary role. Further research is needed to identify the extent and fungal preference of X. perforans in NSW forests to yield important insights for forest biosecurity, pest and disease management and a confirmation that these fungi are most likely transmitted between hosts by beetles (Aoki et al. 2019; Hulcr and Stelinski 2017; Procter et al. 2020). This is currently difficult, with negligible tree colonisation by Xyleborus spp. in pine plantations in NSW following abundant rainfall in the past three years since the initial 2019 observations when the trees were drought-stressed.
Ambrosia beetle-fungus symbioses are heterogenous and highly dynamic (Hulcr and Stelinski 2017; Kostovcik et al. 2015; Musvuugwa et al. 2015). As such, evidence for phylogenetic co-evolution and symbiont specificity is lacking (Kostovcik et al. 2015; Six 2012) and many fungal taxa may still await discovery. It is possible that other fungi of interest may have been present but were rare or missed by the single-spore culturing methods (Linnakoski et al. 2012) or the small number of pine plantation sites sampled. It is likely that an increase in sites and hosts sampled may have led to the detection of more fungal associates of Xyleborus beetles. Nevertheless, based on isolation frequency alone, matched with their association to a secondary (beetle) pest that prefers dead host material, R. deltoideospora and S. pseudoabietina do not appear to be aggressive fungi due to their low recovery rate across three sample types. Perhaps their most important role is as a nutritional symbiont in the X. perforans lifecycle, as has been previously described (Bateman et al. 2016; Hulcr and Stelinski 2017; Kasson et al. 2013; Mayers et al. 2022; Saucedo-Carabez et al. 2018).
Conclusion
The changing climate represents many challenges for ecological interactions and forest health; a key being more suitable habitats for biological invaders like ambrosia beetles which are considered to be one of the world’s top invaders of new ecological niches (Urvois et al. 2021). The extreme drought observed in this study prior to forest health surveys in February 2019 may have provided the opportunity for Xyleborus beetles to reach epidemic proportions and atypically colonise living trees. Modelling suggests such conditions are likely to become more common (Carnegie et al. 2022). While this study did not identify any new fungal pathogens for forest management, it did confirm that X. perforans forms promiscuous associations with fungi in NSW pine forests. This was demonstrated by the identification of five fungal associates including F. parceramosum. F. aff. solani, O. ips, S. pseudoabietina and R. deltoideospora. While O. ips causes significant economic impacts, the other species also belong to important fungal clades that contain significant pathogens that are vectored by aggressive primary ambrosia beetle pests. Additionally, this study’s detection of F. parceramosum, S. pseudoabietina, and R. deltoideospora represents the first report of their association with a Xyleborus beetle in Australia and the latter appears specific to beetle mycangia. These results demonstrate the potential threat of this ambrosia beetle to forest ecosystems and the need for greater understanding of the ecological relationships that exist in Australian forests and the continued surveillance of the diversity and identities of their fungal associations. Further detailed insect-fungal studies are essential to better understand biosecurity and forest health risks for Australia’s natural and planted forests. If new exotic pests or pathogens were to establish in Australia, the economic and biological losses could be significant (Carnegie and Nahrung 2019; Wingfield et al. 2001).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Alamouti S, Tsui CKM, Breuil C (2009) Multigene phylogeny of filamentous ambrosia fungi associated with ambrosia and bark beetles. Mycol Res 113(8):822–835. https://doi.org/10.1016/j.mycres.2009.03.003
Alamouti SM, Kim JJ, Breuil C (2006) A new Leptographium species associated with the northern spruce engraver, Ips perturbatus, in western Canada. Mycologia 98:149–160. https://doi.org/10.1080/15572536.2006.11832722
Aoki T, Smith JA, Kasson MT, Freeman S, Geiser DM, Geering ADW, O'Donnell K (2019) Three novel Ambrosia Fusarium Clade species producing clavate macroconidia known (F. floridanum and F. obliquiseptatum) or predicted (F. tuaranense) to be farmed by Euwallacea spp. (Coleoptera: Scolytinae) on woody hosts. Mycologia 111(6):919–935. https://doi.org/10.1080/00275514.2019.1647074
Australian Bureau of Agricultural and Research Economics and Sciences (2019) Australia's forests at a glance 2019: with data to 2017–18
Bateman C, Šigut M, Skelton J, Smith KE, Hulcr J (2016) Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ Entomol 45(4):883–890. https://doi.org/10.1093/ee/nvw070
Batra LR (1963) Ecology of ambrosia fungi and their dissemination by beetles. Trans Kans Acad Sci 66(2):213–236. https://doi.org/10.2307/3626562
Batra LR (1966) Ambrosia fungi: extent of specificity to ambrosia beetles. Science 153(3732):193–195. https://doi.org/10.1126/science.153.3732.193
Biedermann PHW, Klepzig KD, Taborsky M (2009) Fungus cultivation by ambrosia beetles: behavior and laboratory breeding success in three Xyleborine species. Environ Entomol 38:1096–1105. https://doi.org/10.1111/1574-6941.12026
Carnegie A, Nahrung H (2019) Post-border forest biosecurity in Australia: response to recent exotic detections, current surveillance and ongoing needs. Forests 10(4):336. https://doi.org/10.3390/f10040336
Carnegie AJ, Cant RG, Eldridge RH (2008) Forest health surveillance in New South Wales, Australia. Aust For 71(3):164–176. https://doi.org/10.1080/00049158.2008.10675031
Carnegie AJ, Kathuria A, Nagel M, Mitchell PJ, Stone C, Sutton M (2022) Current and future risks of drought-induced mortality in Pinus radiata plantations in New South Wales. Australia Australian Forestry 85(4):161–177. https://doi.org/10.1080/00049158.2022.2145722
Carrillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC, Peña JE (2014) Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol 63:54–62. https://doi.org/10.1111/ppa.12073
Chang R, Duong TA, Taerum SJ, Wingfield MJ, Zhou X, de Beer ZW (2017) Ophiostomatoid fungi associated with conifer-infesting beetles and their phoretic mites in Yunnan. China Mycokeys 28(28):19–64. https://doi.org/10.3897/mycokeys.28.21758
Cruz LF, Menocal O, Mantilla J, Ibarra-Juarez LA, Carrillo D (2019) Xyleborus volvulus (Coleoptera: Curculionidae): biology and fungal associates. Appl Environ Microbiol 85(19):1–11. https://doi.org/10.1128/AEM.01190-19
Cruz LF, Rocio SA, Duran LG, Menocal O, Garcia-Avila CDJ, Carrillo D (2018) Developmental biology of Xyleborus bispinatus (Coleoptera: Curculionidae) reared on an artificial medium and fungal cultivation of symbiotic fungi in the beetle’s galleries. Fungal Ecol 35:116–126. https://doi.org/10.1016/j.funeco.2018.07.007
Custódio FA, Pereira OL (2023) First report of Neocosmospora ipomoeae causing basal stem rot on Adenium obesum. Crop Prot 164:106–138. https://doi.org/10.1016/j.cropro.2022.106138
Department of Agriculture, Fisheries and Forestry ABARES (2018) Australia's State of the Forests Report. Montreal Process Implementation Group for Australia and National Forest Inventory Steering Committee. Retrieved 13 July 2022 from https://www.agriculture.gov.au/abares/forestsaustralia/sofr/sofr-2018
Diehl JMC, Keller A, Biedermann PHW (2023) Comparing the succession of microbial communities throughout development in field and laboratory nests of the ambrosia beetle Xyleborinus saxesenii. Front Microbiol 14:1151208–1151208
Dreaden TJ, Davis JM, de Beer ZW, Ploetz RC, Soltis PS, Wingfield MJ, Smith JA (2014) Phylogeny of ambrosia beetle symbionts in the genus Raffaelea. Fungal Biol 118:970–978. https://doi.org/10.1016/j.funbio.2014.09.001
Elliott HJ, Ohmart CP, Wylie FR (1998) Insect pests of Australian forests: ecology and management. Inkata Press
Flechtmann CAH, Ottati ALT, Berisford CW (2001) Ambrosia and bark beetles (Scolytidae: Coleoptera) in pine and eucalypt stands in southern Brazil. For Ecol Manage 142(1):183–191. https://doi.org/10.1016/S0378-1127(00)00349-2
Forestry Corporation of New South Wales (2022) Sustainability Report 2021–2022. Retrieved 16 June 2023 from https://app.powerbi.com/view?r=eyJrIjoiZTU2OGVhYjAtZjEwNi00YzRjLTg2MDAtZjg0NWU1Y2E3MWZmIiwidCI6IjdlODcyMjA5LWY3MGItNDU3OC1hNzk5LTA4YTdjZjAzODI3NSJ9&pageName=ReportSectionb205a71ea08442149221
Forrest WG (1973) Biological and economic production in radiata pine plantations. J Appl Ecol 10(1):259–267. https://doi.org/10.2307/2404729
Francke-Grossman H (1967) Ectosymbiosis in wood-inhabiting insects. In S. Henry (Ed.), Symbiosis (Vol. 2, pp. 141–203). Academic Press
Gebhardt H, Weiss M, Oberwinkler F (2005) Dryadomyces amasae: A nutritional fungus associated with ambrosia beetles of the genus Amasa (Coleoptera: Curculionidae, Scolytinae). Mycol Res 109(6):687–696. https://doi.org/10.1017/S0953756205002777
Guarnaccia V, Martino I, Brondino L, Gullino ML (2022) Paraconiothyrium fuckelii, Diaporthe eres and Neocosmospora parceramosa causing cane blight of red raspberry in Northern Italy. Journal of Plant Pathology 104(2):683–698. https://doi.org/10.1007/s42161-022-01068-4
Harrington TC (2005) Ecology and evolution of mycophagous bark beetles and their fungal partners. In: Vega F, Blackwell M (eds) Insect-Fungal Associations. Oxford University Press, pp 257–291
Harrington TC, Fraedrich SW, Aghayeva DN (2008) Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 104:399–404
Hood I, Ramsden M (1997) Sapstain and decay following fire in stands of Pinus elliottii var. elliottii near Beerburrum, south east Queensland. Australian Forestry 60(1):7–15
Hulcr J, Stelinski LL (2017) The ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol 62(1):285–303. https://doi.org/10.1146/annurev-ento-031616-035105
Joseph R, Keyhani NO (2021) Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Appl Microbiol Biotechnol 105(9):3393–3410. https://doi.org/10.1007/s00253-021-11268-0
Katoh K, Misawa K, Kuma Ki, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. https://doi.org/10.1093/nar/gkf436
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kasson MT, O’Donnell K, Rooney AP, Sink S, Ploetz RC, Ploetz JN, Konkol JL, Carrillo D, Freeman S, Mendel Z, Smith JA, Black AW, Hulcr J, Bateman C, Stefkova K, Campbell PR, Geering ADW, Dann EK, Eskalen A, Mohotti K, Short DPG, Aoki T, Fenstermacher KA, Davis DD, Geiser DM (2013) An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet Biol 56:147–157. https://doi.org/10.1016/j.fgb.2013.04.004
Klepzig KD, Six DL (2004) Bark beetle-fungal symbiosis: context depedency in complex associations. Symbiosis 37:189–205
Kostovcik M, Bateman CC, Kolarik M, Stelinski LL, Jordal BH, Hulcr J (2015) The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J 9(1):126–138. https://doi.org/10.1038/ismej.2014.115
Kubono T, Ito S-I (2002) Raffaelea quercivora sp. nov. associated with mass mortality of Japanese oak, and the ambrosia beetle (Platypus quercivorus). Mycoscience 43(3):255–260. https://doi.org/10.1007/S102670200037
Li Y, Huang Y-T, Kasson MT, Macias AM, Skelton J, Carlson PS, Yin M, Hulcr J (2018a) Specific and promiscuous ophiostomatalean fungi associated with Platypodinae ambrosia beetles in the southeastern United States. Fungal Ecol 35:42–50. https://doi.org/10.1016/j.funeco.2018.06.006
Li Y, Ruan Y, Kasson MT, Stanley EL, Gillett CPDT, Johnson AJ, Zhang M, Hulcr J (2018b) Structure of the ambrosia beetle (Coleoptera: Curculionidae) mycangia revealed through micro-computed tomography. J Insect Sci 18(5):13. https://doi.org/10.1093/jisesa/iey096
Li Y, Ruan YY, Stanley EL, Skelton J, Hulcr J (2019) Plasticity of mycangia in Xylosandrus ambrosia beetles. Insect Science 26(4):732–742. https://doi.org/10.1111/1744-7917.12590
Linnakoski R, Wilhelm de Beer ZB, Niemelä P, Wingfield MJ (2012) Associations of conifer-infesting bark beetles and fungi in Fennoscandia. InSects 3(1):200–227. https://doi.org/10.3390/insects3010200
Lynch SC, Twizeyimana M, Mayorquin JS, Wang DH, Na F, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A (2016) Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.-two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108(2):313–329. https://doi.org/10.3852/15-063
Malacrinò A, Rassati D, Schena L, Mehzabin R, Battisti A, Palmeri V (2017) Fungal communities associated with bark and ambrosia beetles trapped at international harbours. Fungal Ecol 28:44–52
Matusick G, Eckhardt LG (2010) The pathogenicity and virulence of four Ophiostomatoid fungi on young Longleaf pine trees. Can J Plant Path 32(2):170–176. https://doi.org/10.1080/07060661.2010.484222
Mayers CG, Harrington TC, Biedermann PHW (2022) Mycangia define the diverse ambrosia beetle-fungus symbioses. In: Schultz TR, Gawne R, Peregrine PN (eds) The Convergent Evolution of Agriculture in Humans and Insects. The MIT Press, Cambridge, MA, pp 105–142
Menocal O, Cruz LF, Kendra PE, Berto M, Carrillo D (2023) Flexibility in the ambrosia symbiosis of Xyleborus bispinatus. Front Microbiol 14:1110474–1110474
Morales-Rodríguez C, Sferrazza I, Aleandri MP, Dalla Valle M, Speranza S, Contarini M, Vannini A (2021) The fungal community associated with the ambrosia beetle Xylosandrus compactus invading the mediterranean maquis in central Italy reveals high biodiversity and suggests environmental acquisitions. Fungal Biol 125:12–24. https://doi.org/10.1016/j.funbio.2020.09.008
Musvuugwa T, de Beer ZW, Duong TA, Dreyer LL, Oberlander KC, Roets F (2015) New species of ophiostomatales from scolytinae and platypodinae beetles in the Cape Floristic Region, including the discovery of the sexual state of Raffaelea. Antonie Van Leeuwenhoek 108(4):933–950. https://doi.org/10.1007/s10482-015-0547-7
Nkuekam GK, Wilhelm De Beer Z, Wingfield MJ, Mohammed C, Carnegie AJ, Pegg GS, Roux J (2011) Ophiostoma species (Ophiostomatales, Ascomycota), including two new taxa on eucalypts in Australia. Aust J Bot 59(3):283–297. https://doi.org/10.1071/BT10231
Popa V, Déziel E, Lavallée R, Bauce E, Guertin C (2012) The complex symbiotic relationships of bark beetles with microorganisms: a potential practical approach for biological control in forestry. Pest Manag Sci 68(7):963–975. https://doi.org/10.1002/ps.3307
Procter M, Nel WJ, Marincowitz S, Crous PW, Wingfield MJ (2020) A new species of Raffaelea from beetle-infested Leucaena leucocephala. Fungal Syst Evol 6:305–314. https://doi.org/10.3114/fuse.2020.06.16
Rabaglia RJ (2005) The validity of Xyleborus impressus Eichhoff (Coleoptera: Curculionidae: Scolytinae) as distinct from Xyleborus ferrugineus (Fabricius). Coleopt Bull 59(2):261–266. https://doi.org/10.1649/768
Rassati D, Marini L, Malacrinò A (2019) Acquisition of fungi from the environment modifies ambrosia beetle mycobiome during invasion. PeerJ (San Francisco, CA) 7:e8103–e8103. https://doi.org/10.7717/peerj.8103
Salman M, Mahmoud R, Fadda Z, Alabdallah O, Najjar K, Radwan J, Abuamsha R (2019) First report of Fusarium euwallaceae on avocado trees in Palestine. Archiv Für Phytopathologie Und Pflanzenschutz 52(9–10):930–937. https://doi.org/10.1080/03235408.2019.1682904
Sandoval-Denis M, Lombard L, Crous PW (2019) Back to the roots: a reappraisal of Neocosmospora. Persoonia 43(1):90–185. https://doi.org/10.3767/persoonia.2019.43.04
Saucedo-Carabez JR, Ploetz RC, Konkol JL, Carrillo D, Gazis R (2018) Partnerships between ambrosia beetles and fungi: lineage-specific promiscuity among vectors of the Laurel Wilt pathogen, Raffaelea lauricola. Microb Ecol 76(4):925–940. https://doi.org/10.1007/s00248-018-1188-y
Saucedo JR, Ploetz RC, Konkol JL, Ángel M, Mantilla J, Menocal O, Carrillo D (2017) Nutritional symbionts of a putative vector, Xyleborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis 75(1):29–38. https://doi.org/10.1007/s13199-017-0514-3
Six DL (2003) A comparison of mycangial and phoretic fungi of individual mountain pine beetles. Can J for Res 33(7):1331–1334. https://doi.org/10.1139/x03-047
Six DL (2012) Ecological and evolutionary determinants of bark beetle - Fungus symbioses. InSects 3(1):339–366. https://doi.org/10.3390/insects3010339
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stone C, Goodyer G, Sims K, Penman T, Carnegie A (2010) Beetle assemblages captured using static panel traps within New South Wales pine plantations: Beetle assemblages in NSW pine plantations. Aust J Entomol 49(4):304–316. https://doi.org/10.1111/j.1440-6055.2010.00769.x
Stone C, Simpson JA (1987) Influence of Ips grandicollis on the incidence and spread of blue stain fungi in Pinus elliottii billets in north-eastern New South Wales. Aust For 50(2):86–94. https://doi.org/10.1080/00049158.1987.10674500
Thu PQ, Quang DN, Chi NM, Hung TX, Van Binh L, Dell B (2021) New and emerging insect pest and disease threats to forest plantations in Vietnam. Forests 12(10):1301. https://doi.org/10.3390/f12101301
Trollip C, Carnegie AJ, Dinh Q, Kaur J, Smith D, Mann R, Rodoni B, Edwards J (2021) Ophiostomatoid fungi associated with pine bark beetles and infested pines in south-eastern Australia, including Graphilbum ipis-grandicollis sp. nov. IMA Fungus 12(1):24. https://doi.org/10.1186/s43008-021-00076-w
Trollip C, Kaur J, Piper AM, Martoni F, Mann R, Dinh Q, Carnegie AJ, Rodoni B, Edwards J (2022) Modular, multi-barcode amplicon sequencing for improved species-level detection of fungal phytopathogens: a case study of pipeline establishment targeting the Ophiostomatales. Environmental DNA. https://doi.org/10.1002/edn3.368
Urvois T, Auger-Rozenberg MA, Roques A, Rossi JP, Kerdelhue C (2021) Climate change impact on the potential geographical distribution of two invading Xylosandrus ambrosia beetles. Sci Rep 11:1339. https://doi.org/10.1038/s41598-020-80157-9
Vaartaja O (1967) The common fungal associates of bark beetle, Ips grandicollis in Pinus radiata in South Australia. Aust For Res 2(4):40–43
Webber JF (1990) Relative effectiveness of Scolytus scolytus, S. multistriatus and S. kirschi as vectors of Dutch elm disease. Euro J Forest Pathol 20(3):184–192. https://doi.org/10.1111/j.1439-0329.1990.tb01129.x
Wingfield MJ, Slippers B, Roux J, Wingfield BD (2001) Worldwide movement of exotic forest fungi, especially in the tropics and the southern hemisphere. Bioscience 51(2):134–140. https://doi.org/10.1641/0006-3568(2001)051[0134:WMOEFF]2.0.CO;2
Wylie FR, Peters B, DeBaar M, King J, Fitzgerald C (1999) Managing attack by bark and ambrosia beetles (Coleoptera: Scolytidae) in fire-damaged Pinus plantations and salvaged logs in Queensland, Australia. Aust For 62(2):148–153. https://doi.org/10.1080/00049158.1999.10674776
Yousuf F, Gurr GM, Carnegie AJ, Bedding RA, Bashford R, Gitau CW, Nicol HI (2014) The bark beetle, Ips grandicollis, disrupts biological control of the woodwasp, Sirex noctilio, via fungal symbiont interactions. FEMS Microbiol Ecol 88(1):38–47. https://doi.org/10.1111/1574-6941.12267
Acknowledgements
Peter Gillespie, Ainsley Seago and Manon Griffith for beetle identifications. Forestry Corporation of NSW for access to pine plantations.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
ZIM, AJC, KS, CT and DIG conceived the idea of the study, and all authors contributed to the study design. AJC provided samples, access to NSW pine plantations, and supervised additional collections by ZIM. ZIM performed isolations, pathogenicity testing, sequencing and data analysis with contribution and supervision from KS and CT. ML conducted preliminary phylogenetic investigations for the Fusarium isolates. Further phylogenetic analyses for species identification of isolates belonging to the four major fungal taxonomic groups were performed by ZIM and CT. ZIM wrote the draft manuscript, with editing and revision by AJC, KS, CT and DIG to produce the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahony, Z., Scarlett, K., Carnegie, A. et al. Fungi associated with the ambrosia beetle Xyleborus perforans (Coleoptera: Curculionidae: Scolytinae) on drought-stressed Pinus in New South Wales, Australia. Australasian Plant Pathol. 53, 51–62 (2024). https://doi.org/10.1007/s13313-023-00952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-023-00952-6