Abstract
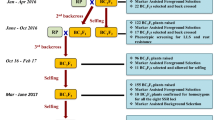
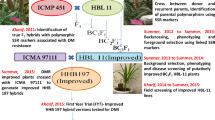
Sorghum downy mildew (SDM), caused by Peronosclerospora sorghi is one of the important diseases in maize. Here, we illustrate the transfer of two QTLs for SDM on chromosome 3 and 6 from UMI 936 (w) into an elite maize inbred line UMI 79 through marker-assisted backcross breeding (MABC). For foreground selection, plants carrying heterozygote allele for the QTLs for SDM were selected from F1 and three back cross (BC1-BC3) generations using simple sequence repeat (SSR) markers linked to the targeted QTLs region of SDM. Consequently, foreground positive plants from BC3F1 generation selfed to produce BC3F2 and plants carrying the favorable alleles for the QTLs for SDM were selected followed by stringent phenotyping against SDM and advanced to BC3F3. As a result, two improved lines (79/936–1–7-7-7-46-46 and 79/936–1–7-7-10-80-80) possessing QTLs for SDM were obtained. Background selection using 146 SSR markers distributed evenly across the maize genome revealed 92.45% and 89.68% recovery of recurrent parent genome (RPG) in two improved lines. Phenotypic characterization of the improved lines showed more than 90% of recovery of the recurrent parents in morphological traits and a resistance to SDM. In conclusion, the newly developed SDM resistant inbred lines are a potential source for improving SDM resistance in maize breeding programs.




Similar content being viewed by others
References
Agrama HA, Moussa ME, Naser ME, Tarek MA, Ibrahim AH (1999) Mapping of QTL for downy mildew resistance in maize. Theor Appl Genet 99(3–4):519–623
Anonymous (1991) “IBPGR descriptor for maize” in international wheat and maize improvement center. International Board for Plant Genetic, Mexico City
Chandran S, Pukalenthy B, Adhimoolam K, Manickam D, Sampathrajan V, Chocklingam V, Hossain F (2019) Marker-assisted selection to pyramid the opaque-2 (o2) and β-carotene (crtRB1) genes in maize. Front Genet 10:859
Craig J, Bockholt AJ, Frederiksen RA, Zuber MS (1977) Reaction of important corn inbred lines to Sclerospora sorghi. Plant Dis 61(7):563–564
Dellaporta SL, Wood J, Hicks JB (1983) Isolation of DNA from higher plants. Plant Mol Biol Report 4:19–21
George ML, Prasanna BM, Rathore RS, Setty TA, Kasim F, Azrai M, Vasal S, Balla O, Hautea D, Canama A, Regalado E (2003) Identification of QTLs conferring resistance to downy mildews of maize in Asia. Theor Appl Genet 107(3):544–551
Jorboe SG, Beavis WD, Openshaw SJ (1994) Prediction of responses to selection in marker-assisted backcross programs by computer simulation. In: Second International Conference on Plant Genome, Scherago International Inc (Vol. 38)
Knapp SJ (1998) Marker-assisted selection as a strategy for increasing the probability of selecting superior genotypes. Crop Sci 38(5):1164–1174
Lal S, Singh IS (1984) Breeding for resistance to downy mildews and stalk rots in maize. Theor Appl Genet 69:111–119
Lohithaswa HC, Jyothi K, Kumar KRS, Puttaramanaik S (2015) HittalmaniIdentification and introgression of QTLs implicated in resistance to sorghum downy mildew (Peronosclerospora sorghi (Weston and Uppal) C. G. Shaw) in maize through marker-assisted selection. J Genet 94:741–748
Murdia LK, Wadhwani R, Wadhawan N, Bajpai P, Shekhawat S (2016) Maize utilization in India: an overview. A J Food Nutr 4(6):169–176
Naidoo R, Watson GM, Derera J, Tongoona P, Laing MD (2012) Marker-assisted selection for low phytic acid (lpa1-1) with single nucleotide polymorphism marker and amplified fragment length polymorphisms for background selection in a maize backcross breeding programme. Mol Breed 30(2):1207–1217
Nair SK, Prasanna BM, Garg A, Rathore RS, Setty TA, Singh NN (2005) Identification and validation of QTLs conferring resistance to sorghum downy mildew (Peronosclerospora sorghi) and Rajasthan downy mildew (P. heteropogoni) in maize. Theor Appl Genet 110(8):1384–1392
Pingali PL, Pandey S (2001) Meeting world maize needs: technological opportunities and priorities for the public sector, 1999/2000.World Maize Facts and Trends
Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001) Quality protein maize. Curr Sci 25:1308–1319
Pukalenthy B, Manickam D, Chandran S, Adhimoolam K, Sampathrajan V, Rajasekaran R, Arunachalam K, Ganapathyswamy H, Chocklingam V, Muthusamy V, Hossain F (2019) Incorporation of opaque-2 into ‘UMI 1200’, an elite maize inbred line, through marker-assisted backcross breeding. Biotechnol and Biotechnol Equip, pp 1-10
Ribaut JM, Hoisington D (1998) Marker-assisted selection: new tools and strategies. Trends Plant Sci 3(6):236–239
Rosegrant MR, Ringler C, Sulser TB, Ewing M, Palazzo A, Zhu T, Nelson GC, Koo J, Robertson R, Msangi S, Batka M (2009) Agriculture and food security under global change: prospects for 2025/2050. International Food Policy Research Institute, Washington, DC, pp 145–178
Sabry A, Jeffers D, Vasal SK, Frederiksen R, Magill C (2006) A region of maize chromosome 2 affects response to downy mildew pathogens. Theor Appl Genet 113(2):321–330
Sireesha Y, Velazhahan R (2016) Biological control of downy mildew of maize caused by Peronosclerospora sorghi under environmentally controlled conditions. J Appl Nat Sci 8(1):279–283
Vasistha NK, Balasubramaniam A, Mishra VK, Srinivasa J, Chand R, Joshi AK (2017) Molecular introgression of leaf rust resistance gene Lr34 validates enhanced effect on resistance to spot blotch in spring wheat. Euphytica 213(12):262
Zhao X, Tan G, Xing Y, Wei L, Chao Q, Zuo W, Lubberstedt T, Xu M (2012) Marker-assisted introgression of qHSR1 to improve maize resistance to head smut. Mol Breed 30(2):1077–1088
Acknowledgements
This work was financially supported by University Grants Commission (UGC), New Delhi (F.No.40-288/2011(SR) dt.29.6.2011) and Department of Biotechnology (DBT), Government of India (GOI), under the project entitled “Molecular tagging of downy mildew resistance in maize and introgression in to the elite inbred lines” (BT/PR108910/GBD/27/111/2008 dt. 02.06.2008).
Author information
Authors and Affiliations
Contributions
NS and NKG conceived and designed the methods and experiments. KS, PA, VS and NKG constructed backcross progenies and managed the fieldwork. KS, PA, VS and MD conducted phenotype and genotype analyses. VP provided suggestions on experiments. AK, SD, KS and NS drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Sumathi, K., Ganesan, K.N., Aarthi, P. et al. Introgression of QTLs determining sorghum downy mildew (SDM) resistance into elite maize line UMI 79 through marker-assisted backcross breeding (MABC). Australasian Plant Pathol. 49, 159–165 (2020). https://doi.org/10.1007/s13313-020-00686-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00686-9