Abstract
Deep brain stimulation (DBS) represents one of the major clinical breakthroughs in the age of translational neuroscience. In 1987, Benabid and colleagues demonstrated that high-frequency stimulation can mimic the effects of ablative neurosurgery in Parkinson’s disease (PD), while offering two key advantages to previous procedures: adjustability and reversibility. Deep brain stimulation is now an established therapeutic approach that robustly alleviates symptoms in patients with movement disorders, such as Parkinson’s disease, essential tremor, and dystonia, who present with inadequate or adverse responses to medication. Currently, stimulation electrodes are implanted in specific target regions of the basal ganglia–thalamic circuit and stimulation pulses are delivered chronically. To achieve optimal therapeutic effect, stimulation frequency, amplitude, and pulse width must be adjusted on a patient-specific basis by a movement disorders specialist. The finding that pathological neural activity can be sampled directly from the target region using the DBS electrode has inspired a novel DBS paradigm: closed-loop adaptive DBS (aDBS). The goal of this strategy is to identify pathological and physiologically normal patterns of neuronal activity that can be used to adapt stimulation parameters to the concurrent therapeutic demand. This review will give detailed insight into potential biomarkers and discuss next-generation strategies, implementing advances in artificial intelligence, to further elevate the therapeutic potential of DBS by capitalizing on its modifiable nature. Development of intelligent aDBS, with an ability to deliver highly personalized treatment regimens and to create symptom-specific therapeutic strategies in real-time, could allow for significant further improvements in the quality of life for movement disorders patients with DBS that ultimately could outperform traditional drug treatment.
Similar content being viewed by others
Introduction and Scope of the Article
Chronic high-frequency stimulation is an effective treatment for patients with movement disorders, such as Parkinson’s disease (PD) [1,2,3], dystonia [4,5,6,7,8], and essential tremor [9,10,11,12,13]. Therefore, functional neurosurgery utilizing precise stereotactic targeting is performed to implant electrodes (see Fig. 1a for exemplar DBS electrodes in the subthalamic nucleus), which contain up to eight stimulation contacts. During implantation surgery, electrophysiological recordings can be performed to identify target-specific patterns of spiking or population activity to verify correct positioning of the electrode [14,15,16,17,18]. After the electrode implantation, lead externalization [19] provides a unique opportunity to record neural population activity as local field potentials (LFP; a population-level measure of synaptic currents and local spiking activity [20, 21]; see Fig. 1b) using external amplification and recording equipment [20]. Intraoperative and postoperative invasive neurophysiology studies in DBS patients have contributed significantly to our understanding of movement disorders at the network level [22,23,24,25,26]. Most importantly, they have inspired a novel concept of stimulation called closed-loop adaptive DBS (aDBS) which aims to move from a chronic continuous stimulation setting to a demand-dependent approach. The present article aims to summarize the utility of specific electrophysiological findings for aDBS in movement disorders patients, reviews present data on aDBS implementations, and concludes with an outlook on the therapeutic potential of artificial intelligence–based strategies to improve clinical outcome in aDBS. Of note, this review will focus on findings derived from human DBS patients, because their potential clinical utility is easier to translate, when compared to animal studies. Nevertheless, animal as well as computational simulation studies remain crucial to further elucidate the mechanism of DBS and the underlying physiological response.
Pathophysiological Neural Activity in DBS Patients with Parkinson’s Disease
Beta Activity as Hallmark of Parkinsonian Bradykinesia and Rigidity
The two most common DBS target sites for PD motor symptoms both yield a net inhibitory influence on thalamocortical circuits, (i) the dorsolateral motor part of the subthalamic nucleus (STN; Fig. 1a), which physiologically exerts glutamatergic excitation of (ii) the ventral posterolateral part of the internal pallidum (GPi), which gives rise to GABAergic inhibitory efferents to the thalamus [27, 28]. In human patients, the observation that both pallidal [29,30,31] and subthalamic [20, 31,32,33,34,35] activities demonstrate exaggerated synchronization of multiunit activity and LFP in the beta frequency range (13–35 Hz) has implicated altered local neural synchrony in the pathophysiology of PD [26, 31, 33, 34, 36,37,38,39,40]. Importantly, it was shown that the amplitude of beta activity is correlated with motor sign severity (as measured with UPDRS-III) across a large cohort of patients OFF medication [34, 35, 41]. Furthermore, the alleviation of bradykinesia and rigidity was correlated with a decrease in recorded beta activity [32, 42]. Similarly, subthalamic DBS was demonstrated to suppress beta activity locally [43,44,45,46] and, again, the amount of suppression of local beta activity was correlated with improvement in parkinsonian motor signs [43, 47]. The finding that a biomarker of concurrent parkinsonian motor sign severity can be recorded in real-time through the same electrodes that deliver the therapeutic stimulation has inspired the concept of a demand-dependent adaptive DBS paradigm [48], where the stimulation parameters are adapted directly according to the recorded electrophysiological parkinsonian symptom correlate, closing the loop from recording to stimulation [49, 50]. Importantly, the presence of beta synchronization in the basal ganglia should not be mistaken as PD specific or pathological per se, as studies from other DBS patient groups, such as dystonia [51,52,53] and OCD [54,55,56], have reported peaks of beta activity in the basal ganglia. However, the relative higher amount of low beta (13–20 Hz) synchrony [29, 57] and the correlation with motor sign severity [34, 35] distinguish PD patients from other movement disorders. High beta activity is less related to parkinsonian symptom severity and was proposed to result from hyperdirect pathway communication [35, 58]. Through subitem and subgroup analyses, it was found that beta activity mainly reflects the akinetic/bradykinetic/rigid spectrum of PD motor signs [42, 59], but not tremor, which paradoxically was shown to reduce beta activity [60, 61] in a fashion similar to that of voluntary movement [53, 62]. Nevertheless, it was recently demonstrated that beta activity reflects bradykinesia even during phases of relative desynchronization during continuous movement [63]. Moreover, specific patterns for walking [64,65,66,67] and even riding the bicycle [68] have been reported for beta activity in PD patients. Studies on the spatial distribution of subthalamic beta activity have found a robust overlap between the location of peak beta power with the optimal target location in the dorsolateral STN [69].
Prolonged Beta Bursts May Reflect Pathological States in PD
In the temporal domain, the relative increase in mean beta power can be explained by a prolongation of transient episodes of beta synchronization, so-called beta bursts. Recent observations report that the presence of particularly long beta bursts in the STN [70,71,72] and the internal pallidum [72, 73] are characteristic for the PD OFF state, while shorter duration bursts are similar between PD ON and dystonia [73] and may reflect normal physiology, which may again be attributed to the presence of beta bursts during motor performance [53].
Using Implantable Recording Devices for Long-Term Characterization of Pathological LFP in PD
Most of the abovementioned results were obtained through LFP recordings from externalized DBS electrodes, without the opportunity to collect information regarding the long-term stability of beta activity in PD. The Medtronic PC+S is an experimental sensing-enabled IPG device that allows local field potentials (LFP) to be recorded directly by the implanted pulse generator [74]. Using the PC+S, studies have recently replicated beta activity suppression through DBS [44, 46, 75] and dopaminergic medication, in a systematic evaluation performed up to 8 months post implantation [34]. Again, beta activity correlated significantly with parkinsonian symptom severity, indicating a sustained association of clinical PD symptoms and oscillatory synchronization. Finally, studies using the PC+S have demonstrated changes in neural entropy related to freezing of gait [65] and differential effects of 60 Hz and 140 Hz stimulation on beta activity [76]. During sleep, a complex modulation of beta activity was found that is dependent on the sleep stage [77].
Corticosubthalamic Electrophysiology in PD Research
To investigate pathological oscillations beyond the basal ganglia, whole-head magnetoencephalography was conducted in parallel with subcortical LFP recordings after withdrawal and administration of levodopa as well as during and after cessation of DBS in patients with PD [58, 78,79,80,81,82,83]. Distinct changes in patterns of corticosubthalamic beta oscillatory connectivity were found to be associated with levodopa and DBS responses that again could be utilized to inform adaptive stimulation control. Furthermore, intraoperative electrocorticographic recordings during DBS implantation have revealed higher broadband gamma power and higher post-movement beta synchrony in PD patients, compared to those with hyperkinetic movement disorders [84]. Subsequent reports on ECoG demonstrated that exaggerated beta–gamma phase-amplitude coupling (PAC) is a cortical biomarker of PD symptoms [85,86,87]. Most importantly, they showed that STN DBS leads to a decrease in cortical PAC that predicts symptom improvement [85]. Re-investigation of the same datasets revealed that some of the effects could be explained by an increased sharpness of the beta waveform in the parkinsonian state [88], adding waveform shape to the range of potential cortical biomarker features. Additionally, a recent study comparing movement-related cortical activity in ECoG recordings from DBS patients with that recorded from epilepsy patients demonstrated that PAC is increased persistently during motor performance in PD patients to a greater degree in the low-beta versus high-beta range [89].
Beyond Beta Activity in Parkinson’s Disease
Despite the fundamental impact of the characterization of pathological beta activity in PD, the fact that beta power is suppressed during tremor casts doubt on the usefulness of beta activity as the sole biomarker for adaptive adjustment of stimulation. Interestingly, not only parkinsonian bradykinesia/rigidity but also dopaminergic side effects are reflected in subthalamic LFP patterns, where dyskinesia symptoms were associated with increased low frequency (4–8 Hz) and narrowband gamma (60–90 Hz) synchronization [90, 91], very similar to the activity seen during normal movement [53, 62, 92, 93]. Moreover, additional pathophysiological hallmarks have been identified in PD and recent research suggests that indeed these different biomarkers may signal different aspects of parkinsonian motor signs [50]. The second most prominent LFP biomarker associated with parkinsonism is the presence of so-called high-frequency oscillations (HFO) at ~ 250 Hz, which are not generally attenuated by dopaminergic medication, but rather are shifted toward higher frequencies to ~ 350 Hz [61, 94]. Importantly, it was recently shown that this frequency shift is also a reliable biomarker of tremor, even in the ON medicated state [61]. During motor performance, it was further demonstrated that gamma synchronization is reduced in the hypodopaminergic state [53, 79], which taken together with HFO could be utilized for multispectral classification of motor sign severity in PD.
Concluding Remarks
The association of bradykinesia/rigidity symptoms with beta oscillations in Parkinson’s disease has been replicated in many groups and is likely the single most robust electrophysiological biomarker in any neurodegenerative disease to date that is directly implicated in PD pathophysiology. Nevertheless, the fact that it is suppressed by tremor and therefore cannot be used for tremor severity detection impedes its usefulness as a sole biomarker for aDBS control in tremor-dominant patients. Multispectral combination of different symptom-specific LFP patterns may be a more promising approach to improve treatment strategies for patients with tremor and bradykinesia rigidity symptoms.
Pathophysiological Neural Activity in DBS Patients with Hyperkinetic Movement Disorders
Essential Tremor
Similar biomarker studies have been conducted in parallel in other movement disorders that could benefit from the development of adaptive stimulation paradigms. High-frequency stimulation of ventral intermediate nucleus (VIM) of the thalamus has shown great clinical success in the treatment of essential tremor [10,11,12,13, 95]. Here, robust activity in the tremor frequency that can drive periodic muscle contractions [96,97,98,99,100] has been reported. The underlying multiunit activity was recently dissected in terms of the spatial distribution of efferent and afferent connections within the ventrolateral thalamic nuclei offering both spectral and spatial information for tremor-specific activity [100]. Cortical modulation of beta activity through voluntary movement was also demonstrated in ET patients [89, 101], rendering ECoG a useful addition as a biomarker signal to differentiate voluntary and involuntary movements [102].
Dystonia
In patients with dystonia, it was found that pallidal and subthalamic low-frequency activities in the theta/alpha range (4–12 Hz; subsequently referred to as theta activity) are higher in patients with dystonia when compared to those in PD patients [29, 57, 103] and are associated with involuntary muscle contractions [51, 104, 105]. Importantly, a recent study demonstrated a correlation of dystonic symptom severity with pallidal theta activity in a large cohort of patients with cervical dystonia [51]. The same study found considerable overlap within the posterolateral ventral GPi between the locations of maximal theta power [51] and previously reported optimal target locations for DBS in dystonia [106,107,108,109]. This is interesting, because a study investigating the effects of DBS on pallidal oscillations during DBS found a significant suppression of theta activity in patients with mobile or phasic cervical dystonia [110]. Patients with tonic dystonia did not show this suppression, but are also known to show benefit more slowly, only after months of chronic DBS, when compared to phasic patients [111]. Parallel LFP-MEG recordings found a correlation of dystonic symptoms with decreased pallido-cerebellar coupling [112], hinting toward a pathophysiological implication of the cerebellum in the generation of dystonic symptoms [112,113,114,115,116,117].
Tourette’s Syndrome
Finally, a remarkable similarity was observed between dystonic theta oscillations and the LFP pattern found in patients with Tourette syndrome (TS) [118], another complex neuropsychiatric disorder associated with abrupt involuntary movements called tics. Deep brain stimulation for Tourette syndrome is less established, when compared to PD, ET, and dystonia, but clinical studies are currently on the rise [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135], showing a clear increase in patients undergoing DBS surgery. Studies recording LFPs from the most common Tourette target areas in patients, namely, the centromedian–parafascicular nucleus (CM-Pf; close to the nucleus ventralis oralis internus, Voi) and the GPi, have reported exaggerated theta activity [118, 119, 136,137,138,139,140,141]. In addition to several studies reporting case-specific spectral patterns, a recent report demonstrated that higher preoperative motor tic severity scores in the Yale Global Tic Severity Scale (YGTSS) are associated with higher theta power in both the GPi and the CM/Voi regions across a TS cohort [141]. Moreover, overall preoperative tic severity including both motor and vocal tic scores across the cohort could be predicted from a multivariable linear model that included theta and beta activities from both target regions, further supporting the utility of multispectral and multispatial parameters for clinical state decoding [141]. While the latter study was conducted retrospectively without concurrent monitoring of occurring tics, two cases were reported where theta power correlated directly with the rate of individual tic occurrence in TS [142].
Concluding Remarks
Compared to the hypokinetic state in PD that is associated with beta activity, studies on hyperkinetic disorders found exaggerated theta activity (4–12 Hz) in essential tremor, dystonia, and Tourette’s syndrome. How this apparent shift in frequency may arise with respect to dopaminergic tone or synaptic plasticity remains to be elucidated. Furthermore, it is unknown whether phasic dystonia and tremor-specific activity share pathophysiological mechanisms in the basal ganglia that are differentially modulated through cerebellothalamic loops. Nevertheless, converging evidence supports the utility of theta activity for the adaptation of DBS parameters in adaptive stimulation paradigms. The major challenge will be to identify the time course of clinical changes with respect to changes in theta oscillations. Moreover, theta is known to increase with voluntary movement, highlighting the necessity for identification of voluntary movement for accurate state identification (discussed in the last section of this review). Figure 2 summarizes the pathological and physiological patterns that could be used for adaptive control of therapeutic DBS parameters. Note that specific patterns of LFP activity have also been reported in other DBS indications, such as major depressive disorder [143,144,145] and OCD [54, 145, 146], although these go beyond the scope of the present review article.
Current Technical and Clinical Advances in Deep Brain Stimulation Methodology
In contrast to the rapid progress and technical advances in multimodal pathophysiological biomarker studies and the increase in experimental DBS indications, the development of new therapeutic approaches to the use of DBS itself has been relatively slow. However, some important developments should be highlighted, as they can directly influence the way adaptive stimulation can be implemented in the clinical setting. Most prominently, the use of more than four stimulation contacts has gained a relevant role in the clinical routine today [147,148,149,150,151,152,153,154].
Electrode Design
Some stimulation devices now support electrodes with 8 instead of 4 contacts per hemisphere, either covering a larger trajectory with 8 equidistant contacts or allowing directional stimulation through segmented contacts. Covering a larger trajectory has been shown useful for the treatment of gait disturbance in PD, where it was found that stimulation of the substantia nigra pars reticulata (SNr) that lies ventral to the STN can have significant additive effects on axial motor sign alleviation, when compared to STN stimulation alone [155]. Directional stimulation using segmented leads provides the opportunity to steer current toward the optimal response and away from structures, such as the internal capsule, associated with adverse stimulation-induced side effects that often limit the maximal therapeutic stimulation amplitude [148,149,150, 152, 154]. Thus, directional stimulation can, depending on the exact electrode location, reduce the threshold for therapeutic response and increase that for side effects, leading to an overall improvement in the therapeutic window [149,150,151, 153]. The practical usefulness of this technical advance however is currently limited by the time a specialized neurologist, together with the patient, must devote to conducting a thorough review of the effects of monopolar stimulation. Here, an automatic algorithm to determine the best contact combination could significantly advance the clinical adoption of directional stimulation [156,157,158].
Localizing DBS Electrodes
Directly related, identifying the electrode placement postoperatively has recently become easier than ever, including the use of free open-source Lead-DBS software (www.lead-dbs.org) that can semi-automatically determine the position of DBS electrode contacts in postoperative imaging [159]. Investigating electrode localizations postoperatively in a systematic manner offers the potential to improve the programming procedure and investigation of optimal target locations [107, 160,161,162,163], modulated network connectivity [160, 164, 165], and the mapping of electrophysiological biomarkers [51, 53, 56, 69].
DBS Stimulation Parameters
Clinical observations and systematic studies on the relevance of DBS parameters, such as stimulation frequency and pulse width, are further areas of research that can potentially improve therapeutic outcomes [154]. Here, lower frequency stimulation (60 Hz) has been reported to have beneficial effects on gait disturbance in PD, when compared to higher stimulation frequencies [166,167,168,169]. In essential tremor, stimulation frequencies above 100 Hz are routinely used and reported to elicit the best therapeutic effect [13, 170,171,172]. Unfortunately, speech disturbance and ataxia can arise as therapy-limiting side effects requiring reduction of stimulation intensities in these patients [9, 173,174,175]. Here, reducing pulse widths from conventionally used 60 μs to shorter settings of 30–40 μs has been reported to efficiently alleviate tremor, while increasing the side effect thresholds [176]. This effect has been previously reported in PD and attributed to a more local driving of surrounding axons [177, 178].
Recording Local Field Potentials from Implantable Pulse Generators
As mentioned in the first section, the availability of implantable DBS pulse generators that can record electrophysiological neural activity is a prerequisite for chronic adaptive stimulation. The use of four different fully implantable systems has been reported in biomarker or adaptive DBS studies. The most prevalent device, the Activa PC (Medtronic, Inc.), was made available to a global community of DBS clinicians for research investigations in over 20-dB centers worldwide, enabling electrophysiology studies to be conducted without the necessity of a dedicated physiology laboratory. From this effort, one can primarily conclude that producing an implantable device that is capable of recording voltage fluctuations in deep nuclei poses significant difficulties. High-noise floor, cardioballistic artifacts, and internal clock–related spikes in combination with very low amplitude signals, limited battery life in primary cells, and low storage capacity of the IPGs have been identified by both researchers and manufacturers as the next engineering challenges toward reliable chronic adaptive stimulation [67, 179].
Concluding Remarks
Recent advances in clinically available DBS hardware include new electrode designs that allow directional steering of currents and multiple source/target stimulation. Clinical observations suggest that specific stimulation sites may benefit specific symptoms, e.g., SNr stimulation for gait in addition to STN stimulation for bradykinesia/rigidity. Moreover, stimulation settings can be adjusted to increase the therapeutic window and thereby reduce side effects. First generations of implantable devices that enable chronic recording of brain activity have helped to identify technological challenges that future adaptive stimulation systems will need to tackle.
Physiological Mechanisms of Deep Brain Stimulation
Modulation of Oscillatory Activity
The exact mechanism of DBS remains elusive, but important observations should be highlighted before discussing the potential implementations of adaptive stimulation. A DBS-induced modulation of subcortical and cortical oscillations has been reported frequently and replicated many times [43,44,45,46,47, 53, 58, 71, 75, 76, 85, 87, 88, 110, 180,181,182,183,184,185,186] supporting the hypothesis that modulation of pathological oscillatory activity may underlie some of the therapeutic mechanisms of deep brain stimulation [154, 187]. Further support for this concept comes from combined electrophysiology and neuroimaging experiments: in PD, beta activity in the subthalamic nucleus coincides with the optimal target location for DBS treatment; and in dystonia, pallidal theta activity spatially peaked in previously published DBS targets for alleviation of dystonic symptoms [51, 69]. Given the abovementioned reports of oscillatory synchronization along the corticosubcortical axis, exaggerated beta activity is not a local phenomenon but reflects pathological circuit alterations that can be interpreted in the context of the “communication through coherence” paradigm [188], that is, as changes in the network’s capacity to communicate information. The changes in cortical synchronization [58] and PAC found during STN-DBS [85] and GPi-DBS [189] indicate that DBS exerts its modulatory effect on oscillatory activity along different network nodes.
Modulation of Spiking Activity and Synaptic Plasticity
This circuit modulation can however rely on the alteration of local synaptic properties [190], as suggested by important studies demonstrating DBS-related local suppression of spiking activity for the STN and GPi in PD [14] and the VIM in ET [191]. Interestingly, in pallidal and subthalamic neurons, an increase of GABAergic plasticity was demonstrated that was absent in the largely glutamatergic VIM. Computational firing rate models have recently revealed that suppression of hyperdirect pathway input to the subthalamic nucleus may lead to cognitive reaction time alterations, while the alleviation of clinical motor signs can be attributed to modulation of indirect basal ganglia pathway activity [164].
Concluding Remarks
Deep brain stimulation at high frequencies leads modulation of local and distant neural activities ultimately resulting in net disinhibition of the motor circuit. Oscillatory activity, spike firing, and synaptic plasticity were shown to be altered through DBS and shown to be correlated with clinical DBS effects.
First Implementations of Adaptive Closed-Loop DBS
Parkinson’s Disease
A closed-loop stimulation strategy first was shown to be effective in a non-human primate model of PD via recording in motor cortex and the globus pallidus (GPi) and stimulating in the GPi [192]. The translational nature of this approach recently has attracted many multidisciplinary research groups bringing neurologists, neurosurgeons, engineers, and neuroscientists together with the aim to improve therapeutic outcome in DBS [49, 50, 137, 181, 193,194,195,196,197,198]. The first studies in human PD patients utilized pathological increases in STN beta activity to trigger stimulation with fixed DBS parameters, where stimulation ceased after sufficient beta signal suppression was achieved [194, 199,200,201]. This approach resulted in similar or better symptom alleviation compared with conventional DBS and a reduction of dyskinesia symptoms [181, 195, 201] and stimulation-induced side effects [202]. Most aDBS studies to date only observed aDBS for a short duration during which clinical testing was conducted using externalized equipment in a laboratory environment. The first aDBS study using beta activity to modulate stimulation amplitude with a mobile device has recently been published [181]. That study was carried out during daily activities over a time period of 8 h in 13 akinetic rigid PD patients and reported a significant decrease of stimulation amplitudes during medication ON periods, but did not compare results against chronic non-adaptive DBS. Likewise in PD, the first study of cortical sensing-based aDBS reported promising results in two parkinsonian patients [183]. Unlike the abovementioned studies, the approach here was based on sensing of an ECoG signature of dyskinesia in the gamma frequency band [203]. Thus, whenever this narrowband activity on the cortical level increased, the stimulation amplitude decreased. While therapeutic efficacy was maintained in short-term clinical testing, significant energy savings of 38–45% were reported [183].
Hyperkinetic Movement Disorders
A similar strategy was recently reported to implement aDBS in the CM-Pf target, using the RNS System in a patient with Tourette syndrome, where 5–15-Hz activity was used to trigger stimulation [137]. In ET, few studies have reported use of accelerometery [193] for aDBS, also in combination with ECoG [102], but one study relying only on neural signals has been published to date. The latter has also introduced identification of voluntary behavior to improve stimulation, which will be further discussed below. For dystonia, a single patient was reported to have undergone adaptive DBS in the pallidum, using theta activity as input [204].
Concluding Remarks
While these results provide crucial evidence for the feasibility of aDBS, approaches based on single spectral features recorded mostly from the same locations at which stimulation is delivered have several inherent limitations and do not take advantage of the full potential of adaptive control to improve DBS outcomes. Many important features, such as multispectral patterning or spatial activity distributions, are ignored for the sake of practicality in the absence of both hardware and software solutions that may help clinicians elevate the therapeutic potential of DBS to the next level.
Intelligent Algorithms to Improve aDBS Outcome
Potential Benefit of Cortical Signals for Intelligent Adaptive DBS
In recent years, computational innovations in the field of machine learning, especially deep learning with artificial neural networks, have revolutionized artificial intelligence for high-definition medicine [205], yet these advances are greatly underrepresented in DBS methodology. Adaptive closed-loop stimulation provides a framework to incorporate this approach, but previous applications in pilot studies were limited to a narrow feature space without dynamic adaptation and multiple feature classification [49]. Notwithstanding the impact of piloting aDBS studies for the field of human neural-network neurosurgery, the STN-LFP beta power approach has several limitations that raise questions about its usefulness for adoption in a clinical real-life setting. Most importantly, sensing from the same lead that delivers stimulation is suboptimal in many ways: (i) signal quality deteriorates with stimulation; (ii) bipolar sensing leaves only 2 of 4 available stimulation contacts that necessarily should reside in an optimal target location [181, 194, 195, 200, 202]; (iii) common mode rejection, through which an LFP signal is less perturbed by DBS when sensing contacts are distributed symmetrically around the stimulation contact, may require symmetric current spread, potentially obliterating the additional benefit directional current steering [149, 150, 152]; and (iv) that approach may force DBS target planning to include consideration of biomarker location rather than enabling independent optimal targeting of both sensing and stimulation electrodes. Here, using additional ECoG recordings for closed-loop sensing, which has been reported to be safe [206], could have striking advantages: (i) compatibility with any DBS electrode design; (ii) optimal signal to noise ratio; (iii) placement of the sensing electrode independent of the DBS target (e.g., white matter tracts for tremor); (iv) coverage of large areas (potentially more than one location; many contacts); (v) reduction of stimulation artifacts; and (vi) preservation of the number of potential stimulation contacts in the DBS target.
Multifeature Classification for Adjustment of Stimulation Settings to Concurrent Demand
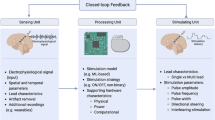
Using single-biomarker thresholds to trigger stimulation also does not allow for adapting stimulation parameters dynamically to behavioral demand. As discussed in the previous sections, emerging evidence suggests that specific DBS parameters can have symptom-specific effects, such as improved freezing of gait through 60-Hz stimulation [166,167,168,169] and stimulation of ventral DBS contacts in the SNr for alleviation of axial motor impairment [155]. Moreover, dyskinesia during medication ON times can limit the therapeutic stimulation amplitude at the cost of better symptom alleviation during OFF times [201], and speech intelligibility and DBS-induced spastic dysarthria can be improved through reduction of stimulation amplitudes [207]. Dynamic adjustment of stimulation parameters to concurrent behavior and demand could therefore have tremendous benefits for the control of symptoms and side effects in patients undergoing deep brain stimulation. A hypothetical multisite recording paradigm in combination with computational models and deep learning algorithms potentially could achieve the following: (i) an electrophysiological classifier that can detect the presence of (a) disease-specific symptoms, such as tremor, bradykinesia, freezing of gait, hypophonia, and dystonia; (b) side effects such as dyskinesia, dysarthria, and ataxia; and (c) normal physiologic function such as voluntary movement, sleep, walking, and speaking. The classifier could give feedback to a (ii) control algorithm that dynamically modulates (a) stimulation amplitude, (b) stimulation contact/direction, (c) stimulation frequency, and (d) pulse widths. While the optimal computational approach remains to be developed, building a hierarchical computational framework to classify multiple pathologic and normal physiological states and predict symptom severity along multiple input (ECoG and LFP) and target domains (tremor, bradykinesia, speech), and across multiple patient cohorts, would enhance the therapeutic benefit of adaptive DBS for movement disorders (Fig. 3).
Deep Neural Networks for Electrophysiology-Based Intelligent Adaptive DBS
Ideally, intelligent aDBS could evolve from a computational model based on deep learning with hierarchically organized artificial neural networks that are optimized to predict the need to adapt DBS stimulation parameters in real-time. Practically, electrophysiological time series data from LFP and ECoG electrodes of each channel can be transformed to the time-frequency domain to produce feature matrices with high temporal resolution from relevant frequency bands (theta, alpha, low beta, high beta, low gamma, high gamma) in addition to raw data and full spectrum features such as total power and variance that have previously been used to decode behavior from neural fields [64, 208,209,210,211,212]. Recurrent neural network approaches (e.g., long short-term memory networks; LSTM [213, 214]) could be used for training on oscillatory neural time series data [215] to simultaneously conduct hierarchical classifications and predictions that can ultimately guide DBS parameter adaptations. This could produce an expandable modular deep neural network architecture that generalizes high-precision feature decoding across patients and recording sites based on ECoG time series with tuning to specific patients based on little or no patient-specific data. Importantly, this approach can learn an almost infinite amount of spectral, spatial, and temporal patterns of activity, given that both concurrent and preceding activities are used to inform the model. This strategy may have significant benefit in the detection of rhythmic activity, such as walking [64, 67], speaking, tremor, [60, 61] or sleep [77], as well as in the prediction of clinical symptom severity [34, 35, 59], where a continuous increase of pathological activity could predict upcoming symptoms more robustly than single time points. This could also account for symptom-specific differences in the time lag to therapeutic response. One of the major limitations of dynamic adaptive stimulation approaches may be the delayed response of certain symptoms to DBS, which have not been studied to a sufficient degree for estimating their potential detrimental influence and thus need to be carefully described in future clinical trials of aDBS. The current frontier in artificial intelligence–based adaptive stimulation is to gather the necessary data for model training. Therefore, the DBS community should seek international multicenter collaborations to share anonymized electrophysiological time series from DBS patients in combination with clinical annotations and/or behavioral data. Multicenter data sharing initiatives provide fundamental advantages over single-center studies: (1) Multiplication of the data that can be used for model training; (2) cross-cohort validation of trained models to secure generalizability. Once the outlined intelligent classifiers are generalizable and established, a clinical implementation could be within reach.
Computational Cost of Deep Learning Solutions for Adaptive Stimulation
The computational cost of model training can be high and increases significantly with the amount of data that training is based on. Importantly, training the models to learn the association of electrophysiological signals with clinical and behavioral patient states can be done offline using high-throughput parallel computing in cluster networks and does not depend on the limited computational power and battery capacity of an IPG. During training, millions of feedback and feedforward passes can be calculated to obtain the optimal set of parameters in combination with hyperparameters and model architectures. The best performing artificial neural networks can then be made available openly to enable researchers to use and further improve them. Once the trained models have proven sufficient predictive performance, they can be stored on an IPG or external hardware device requiring minimal resources, as predictions are single feedforward passes of input data. Data preparation and signal analysis, however, may induce memory demand on implanted devices that could be solved through implemented hardware signal analysis routines. Instead of Fourier or wavelet time frequency transformations to assess frequency-specific signal alterations, hardware filters could divide signals in relevant frequency ranges and amplitude span could be sampled several times per second to reduce computational cost (e.g., sampling hardware filtered 60–90-Hz gamma band activity 20 times per second instead of sampling raw signal at 250 Hz or higher and deriving gamma oscillations through Fourier transform). In conclusion, neither memory nor energy consumption would significantly limit the implementation of deep learning on implantable devices, but researching optimal solutions is only possible with external hardware prototypes.
Conclusion
In the age of artificial intelligence, the field of DBS has entered an era where improvements in device technology have created a gap between user and device capabilities. Our proposed framework implies crucial steps for successful real-life applications of adaptive stimulation and goes above and beyond previous attempts of feature extraction for aDBS. Intelligent aDBS promises significant improvements in quality of life for patients with movement disorders that eventually could outperform traditional pharmacological treatment, due to the highly personalized nature of symptom-specific therapeutic strategies created in real-time. The basal ganglia communicate with a range of motor and non-motor regions [216,217,218]. Decoding circuit-specific target computations [164] could further elucidate the role of the basal ganglia in neurological disease and result in greater improvements in quality of life for patients with movement disorders. Large-scale multicenter studies on artificial intelligence–based methods for behavioral and clinical state decoding represent the frontier in next-generation adaptive stimulation.
References
Schuepbach, W.M., et al., Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med, 2013. 368(7): p. 610–22.
Weaver, F.M., et al., Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama, 2009. 301(1): p. 63–73.
Deuschl, G., et al., A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med, 2006. 355(9): p. 896–908.
Vidailhet, M., et al., Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol, 2007. 6(3): p. 223–9.
Vidailhet, M., et al., Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med, 2005. 352(5): p. 459–67.
Volkmann, J., et al., Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol, 2014. 13(9): p. 875–84.
Volkmann, J., et al., Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol, 2012. 11(12): p. 1029–38.
Kupsch, A., et al., Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med, 2006. 355(19): p. 1978–90.
Alomar, S., et al., Speech and language adverse effects after thalamotomy and deep brain stimulation in patients with movement disorders: A meta-analysis. Mov Disord, 2017. 32(1): p. 53–63.
Flora, E.D., et al., Deep brain stimulation for essential tremor: a systematic review. Mov Disord, 2010. 25(11): p. 1550–9.
Kumar, R., et al., Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology, 2003. 61(11): p. 1601–4.
Schuurman, P.R., et al., A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med, 2000. 342(7): p. 461–8.
Benabid, A.L., et al., Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet, 1991. 337(8738): p. 403–6.
Milosevic, L., et al., Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease. Brain, 2018. 141(1): p. 177–190.
Lipski, W.J., et al., Dynamics of human subthalamic neuron phase-locking to motor and sensory cortical oscillations during movement. J Neurophysiol, 2017. 118(3): p. 1472–1487.
Hutchison, W.D., et al., Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol, 1998. 44(4): p. 622–8.
Vitek, J.L., et al., Intraoperative neurophysiology in DBS for dystonia. Mov Disord, 2011. 26 Suppl 1: p. S31–6.
Vitek, J.L., et al., Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol, 1999. 46(1): p. 22–35.
Rosa, M., et al., Risk of Infection After Local Field Potential Recording from Externalized Deep Brain Stimulation Leads in Parkinson’s Disease. World Neurosurg, 2017. 97: p. 64–69.
Kühn, A.A., et al., The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp Neurol, 2005. 194(1): p. 212–20.
Tachibana, Y., et al., Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci, 2011. 34(9): p. 1470–84.
Brittain, J.S. and P. Brown, Oscillations and the basal ganglia: motor control and beyond. Neuroimage, 2014. 85 Pt 2: p. 637–47.
Brittain, J.S., A. Sharott, and P. Brown, The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci, 2014. 39(11): p. 1951–9.
Jenkinson, N., A.A. Kühn, and P. Brown, Gamma oscillations in the human basal ganglia. Exp Neurol, 2013. 245: p. 72–6.
Jenkinson, N. and P. Brown, New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci, 2011. 34(12): p. 611–8.
Brown, P., Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol, 2007. 17(6): p. 656–64.
Albin, R.L., A.B. Young, and J.B. Penney, The functional anatomy of disorders of the basal ganglia. Trends Neurosci, 1995. 18(2): p. 63–4.
Albin, R.L., A.B. Young, and J.B. Penney, The functional anatomy of basal ganglia disorders. Trends Neurosci, 1989. 12(10): p. 366–75.
Silberstein, P., et al., Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain, 2003. 126(Pt 12): p. 2597–608.
Herz, D.M., et al., Mechanisms Underlying Decision-Making as Revealed by Deep-Brain Stimulation in Patients with Parkinson’s Disease. Curr Biol, 2018. 28(8): p. 1169–1178.e6.
Brown, P., et al., Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci, 2001. 21(3): p. 1033–8.
Kühn, A.A., et al., Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease, in Eur J Neurosci. 2006: France. p. 1956–60.
Brown, P. and D. Williams, Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol, 2005. 116(11): p. 2510–9.
Neumann, W.J., et al., Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s disease. Clin Neurophysiol, 2017. 128(11): p. 2286–2291.
Neumann, W.J., et al., Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord, 2016. 31(11): p. 1748–1751.
Levy, R., et al., Synchronized Neuronal Discharge in the Basal Ganglia of Parkinsonian Patients Is Limited to Oscillatory Activity. 2002.
Hammond, C., H. Bergman, and P. Brown, Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci, 2007. 30(7): p. 357–64.
Brown, P., Bad oscillations in Parkinson’s disease. J Neural Transm Suppl, 2006(70): p. 27–30.
Brown, P., Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord, 2003. 18(4): p. 357–63.
Williams, D., et al., Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain, 2002. 125(Pt 7): p. 1558–69.
Beudel, M., et al., Oscillatory Beta Power Correlates with Akinesia-Rigidity in the Parkinsonian STN. Movement Disorders, 2016.
Kühn, A.A., et al., Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol, 2009. 215(2): p. 380–7.
Kühn, A.A., et al., High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci, 2008. 28(24): p. 6165–73.
Neumann, W.J., et al., Deep Brain Recordings Using an Implanted Pulse Generator in Parkinson’s Disease. Neuromodulation, 2016. 19(1): p. 20–4.
Blumenfeld, Z. and H. Bronte-Stewart, High Frequency Deep Brain Stimulation and Neural Rhythms in Parkinson’s Disease. Neuropsychol Rev, 2015. 25(4): p. 384–97.
Quinn, E.J., et al., Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord, 2015. 30(13): p. 1750–8.
Eusebio, A., et al., Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry, 2011. 82(5): p. 569–73.
Little, S. and P. Brown, What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci, 2012. 1265: p. 9–24.
Meidahl, A.C., et al., Adaptive Deep Brain Stimulation for Movement Disorders: The Long Road to Clinical Therapy. Mov Disord, 2017. 32(6): p. 810–819.
Hoang, K.B., et al., Biomarkers and Stimulation Algorithms for Adaptive Brain Stimulation. Front Neurosci, 2017. 11: p. 564.
Neumann, W.J., et al., A localized pallidal physiomarker in cervical dystonia. Ann Neurol, 2017. 82(6): p. 912–924.
Wang, D.D., et al., Subthalamic local field potentials in Parkinson’s disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol Dis, 2016. 89: p. 213–22.
Lofredi, R., et al., Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson’s disease. Elife, 2018. 7. pii: e31895. https://doi.org/10.7554/eLife.31895.
Rappel, P., et al., Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry, 2018. 8(1): p. 118.
Wojtecki, L., et al., Oscillatory coupling of the subthalamic nucleus in obsessive compulsive disorder. Brain, 2017. 140(9): p. e56.
Accolla, E.A., et al., Reply: Oscillatory coupling of the subthalamic nucleus in obsessive compulsive disorder. Brain, 2017. 140(9): p. e57.
Geng, X., et al., Comparison of oscillatory activity in subthalamic nucleus in Parkinson’s disease and dystonia. Neurobiol Dis, 2017. 98: p. 100–107.
Oswal, A., et al., Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain, 2016.
Neumann, W.J. and A.A. Kühn, Subthalamic beta power-Unified Parkinson’s disease rating scale III correlations require akinetic symptoms. Mov Disord, 2017. 32(1): p. 175–176.
Hirschmann, J., et al., Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin Neurophysiol, 2017. 128(10): p. 2029–2036.
Hirschmann, J., et al., Parkinsonian Rest Tremor Is Associated With Modulations of Subthalamic High-Frequency Oscillations. Mov Disord, 2016. 31(10): p. 1551–1559.
Kühn, A.A., et al., Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain, 2004. 127(Pt 4): p. 735–46.
Steiner, L.A., et al., Subthalamic beta dynamics mirror Parkinsonian bradykinesia months after neurostimulator implantation. Mov Disord, 2017. 32(8): p. 1183–1190.
Fischer, P., et al., Alternating Modulation of Subthalamic Nucleus Beta Oscillations during Stepping. J Neurosci, 2018. 38(22): p. 5111–5121.
Syrkin-Nikolau, J., et al., Subthalamic neural entropy is a feature of freezing of gait in freely moving people with Parkinson’s disease. Neurobiol Dis, 2017. 108: p. 288–297.
Singh, A., et al., Pattern of local field potential activity in the globus pallidus internum of dystonic patients during walking on a treadmill. Exp Neurol, 2011. 232(2): p. 162–7.
Hell, F., et al., Subthalamic oscillatory activity and connectivity during gait in Parkinson’s disease. Neuroimage Clin, 2018. 19: p. 396–405.
Storzer, L., et al., Bicycling suppresses abnormal beta synchrony in the Parkinsonian basal ganglia. Ann Neurol, 2017. 82(4): p. 592–601.
Horn, A., et al., Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp, 2017.
Tinkhauser, G., et al., Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain, 2017. 140(11): p. 2968–2981.
Tinkhauser, G., et al., The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain, 2017. 140(4): p. 1053–1067.
Deffains, M., et al., Longer beta oscillatory episodes reliably identify pathological subthalamic activity in Parkinsonism. Mov Disord, 2018.
Lofredi, R., et al., Pallidal beta bursts in Parkinson’s disease and dystonia. Mov Disord, 2018. Nov 15. https://doi.org/10.1002/mds.27524.
Afshar, P., et al., A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits, 2012. 6: p. 117.
Trager, M.H., et al., Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol Dis, 2016. 96: p. 22–30.
Blumenfeld, Z., et al., Sixty hertz neurostimulation amplifies subthalamic neural synchrony in Parkinson’s disease. PLoS One, 2015. 10(3): p. e0121067.
Urrestarazu, E., et al., Beta activity in the subthalamic nucleus during sleep in patients with Parkinson’s disease. Mov Disord, 2009. 24(2): p. 254–60.
Neumann, W.-J., et al. Kortiko-subthalamische oszillatorische Konnektivität in Patienten mit idiopathischem Parkinson-Syndrom in DGKN. 2014. Berlin: Klin Neurophysiol
Litvak, V., et al., Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci, 2012. 32(31): p. 10541–53.
Litvak, V., et al., Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain, 2011. 134(Pt 2): p. 359–74.
Litvak, V., et al., Optimized beamforming for simultaneous MEG and intracranial local field potential recordings in deep brain stimulation patients. Neuroimage, 2010. 50(4): p. 1578–88.
Hirschmann, J., et al., A direct relationship between oscillatory subthalamic nucleus-cortex coupling and rest tremor in Parkinson’s disease. Brain, 2013. 136(Pt 12): p. 3659–70.
Hirschmann, J., et al., Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. Neuroimage, 2011. 55(3): p. 1159–68.
Crowell, A.L., et al., Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain, 2012. 135(Pt 2): p. 615–30.
de Hemptinne, C., et al., Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci, 2015. 18(5): p. 779–86.
Miocinovic, S., et al., Patterns of Cortical Synchronization in Isolated Dystonia Compared With Parkinson Disease. JAMA Neurol, 2015. 72(11): p. 1244–51.
de Hemptinne, C., et al., Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A, 2013. 110(12): p. 4780–5.
Cole, S.R., et al., Nonsinusoidal Beta Oscillations Reflect Cortical Pathophysiology in Parkinson’s Disease. J Neurosci, 2017. 37(18): p. 4830–4840.
Kondylis, E.D., et al., Movement-related dynamics of cortical oscillations in Parkinson’s disease and essential tremor. Brain, 2016. 139(Pt 8): p. 2211–23.
Alonso-Frech, F., et al., Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain, 2006. 129(Pt 7): p. 1748–57.
Alegre, M., et al., Subthalamic activity during diphasic dyskinesias in Parkinson’s disease. Mov Disord, 2012. 27(9): p. 1178–81.
Brücke, C., et al., Scaling of movement is related to pallidal γ oscillations in patients with dystonia. J Neurosci, 2012. 32(3): p. 1008–19.
Brucke, C., et al., Movement-related synchronization of gamma activity is lateralized in patients with dystonia, in Eur J Neurosci. 2008: France. p. 2322–9.
Ozkurt, T.E., et al., High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol, 2011. 229(2): p. 324–31.
Baizabal-Carvallo, J.F., et al., The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry, 2014. 85(5): p. 567–72.
Pedrosa, D.J., et al., Thalamomuscular coherence in essential tremor: hen or egg in the emergence of tremor? J Neurosci, 2014. 34(43): p. 14475–83.
Pedrosa, D.J., et al., Essential tremor and tremor in Parkinson’s disease are associated with distinct ‘tremor clusters’ in the ventral thalamus. Exp Neurol, 2012. 237(2): p. 435–43.
Kane, A., et al., Enhanced synchronization of thalamic theta band local field potentials in patients with essential tremor. Exp Neurol, 2009. 217(1): p. 171–6.
Marsden, J.F., et al., Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain, 2000. 123 ( Pt 7): p. 1459–70.
Pedrosa, D.J., et al., A functional micro-electrode mapping of ventral thalamus in essential tremor. Brain, 2018.
Air, E.L., et al., Acute effects of thalamic deep brain stimulation and thalamotomy on sensorimotor cortex local field potentials in essential tremor. Clin Neurophysiol, 2012. 123(11): p. 2232–8.
Herron, J.A., et al., Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg, 2017. 127(3): p. 580–587.
Neumann, W.J., et al., Enhanced low-frequency oscillatory activity of the subthalamic nucleus in a patient with dystonia. Mov Disord, 2012. 27(8): p. 1063–6.
Liu, X., et al., The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain, 2008. 131(Pt 6): p. 1562–73.
Liu, X., et al., Different mechanisms may generate sustained hypertonic and rhythmic bursting muscle activity in idiopathic dystonia. Exp Neurol, 2006. 198(1): p. 204–13.
Starr, P.A., et al., Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg, 2006. 104(4): p. 488–501.
Horn, A., et al., Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. Neuroimage, 2017. 150: p. 395–404.
Schonecker, T., et al., Postoperative MRI localisation of electrodes and clinical efficacy of pallidal deep brain stimulation in cervical dystonia. J Neurol Neurosurg Psychiatry, 2015. 86(8): p. 833–9.
Cheung, T., et al., Defining a therapeutic target for pallidal deep brain stimulation for dystonia. Ann Neurol, 2014. 76(1): p. 22–30.
Barow, E., et al., Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain, 2014. 137(Pt 11): p. 3012–24.
Chung, M. and R. Huh, Different clinical course of pallidal deep brain stimulation for phasic- and tonic-type cervical dystonia. Acta Neurochir (Wien), 2016. 158(1): p. 171–80.
Neumann, W.J., et al., Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain, 2015. 138(Pt 7): p. 1894–906.
Neumann, W.J. and A.A. Kühn, Reply: Role of cortico-pallidal connectivity in the pathophysiology of dystonia. Brain, 2016. 139(Pt 9): p. e49.
Cacciola, A., D. Milardi, and A. Quartarone, Role of cortico-pallidal connectivity in the pathophysiology of dystonia, in Brain. 2016: England. p. e48.
Hendrix, C.M. and J.L. Vitek, Toward a network model of dystonia. Ann N Y Acad Sci, 2012. 1265: p. 46–55.
Jinnah, H.A. and E.J. Hess, Evolving concepts in the pathogenesis of dystonia. Parkinsonism Relat Disord, 2017.
Lehericy, S., et al., The anatomical basis of dystonia: current view using neuroimaging. Mov Disord, 2013. 28(7): p. 944–57.
Priori, A., et al., Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci Biobehav Rev, 2013. 37(6): p. 1063–8.
Martinez-Ramirez, D., et al., Efficacy and Safety of Deep Brain Stimulation in Tourette Syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol, 2018. 75(3): p. 353–359.
Akbarian-Tefaghi, L., L. Zrinzo, and T. Foltynie, The Use of Deep Brain Stimulation in Tourette Syndrome. Brain Sci, 2016. 6(3).
Baldermann, J.C., et al., Deep Brain Stimulation for Tourette-Syndrome: A Systematic Review and Meta-Analysis. Brain Stimul, 2016. 9(2): p. 296–304.
Müller-Vahl, K.R., Surgical treatment of Tourette syndrome. Neurosci Biobehav Rev, 2013. 37(6): p. 1178–85.
Savica, R., et al., Deep brain stimulation in tourette syndrome: a description of 3 patients with excellent outcome. Mayo Clin Proc, 2012. 87(1): p. 59–62.
Viswanathan, A., et al., Deep Brain Stimulation for Tourette Syndrome: Target Selection, in Stereotact Funct Neurosurg. 2012, Basel. p. 213–224.
Ackermans, L., et al., Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain, 2011. 134(Pt 3): p. 832–44.
Kaido, T., et al., Deep brain stimulation for Tourette syndrome: a prospective pilot study in Japan. Neuromodulation, 2011. 14(2): p. 123–8; discussion 129.
Ackermans, L., et al., Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J Neurol Neurosurg Psychiatry, 2010. 81(10): p. 1068–72.
Hariz, M.I. and M.M. Robertson, Gilles de la Tourette syndrome and deep brain stimulation. Eur J Neurosci, 2010. 32(7): p. 1128–34.
Bajwa, R.J., et al., Deep brain stimulation in Tourette’s syndrome. Mov Disord, 2007. 22(9): p. 1346–50.
Shahed, J., et al., GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology, 2007. 68(2): p. 159–60.
Ackermans, L., et al., Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord, 2006. 21(5): p. 709–13.
Visser-Vandewalle, V., et al., Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg, 2003. 99(6): p. 1094–100.
Vandewalle, V., et al., Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet, 1999. 353(9154): p. 724.
Welter, M.L., et al., Anterior pallidal deep brain stimulation for Tourette’s syndrome: a randomised, double-blind, controlled trial. Lancet Neurol, 2017. 16(8): p. 610–619.
Welter, M.L., et al., Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol, 2008. 65(7): p. 952–7.
Giorni, A., et al., Single-unit activity of the anterior Globus pallidus internus in Tourette patients and posterior Globus pallidus internus in dystonic patients. Clin Neurophysiol, 2017. 128(12): p. 2510–2518.
Molina, R., et al., Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J Neurosurg, 2017: p. 1–7.
Jimenez-Shahed, J., et al., GPi Oscillatory Activity Differentiates Tics from the Resting State, Voluntary Movements, and the Unmedicated Parkinsonian State. Front Neurosci, 2016. 10: p. 436.
Maling, N., et al., Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PLoS One, 2012. 7(9): p. e44215.
Marceglia, S., et al., Thalamic single-unit and local field potential activity in Tourette syndrome. Mov Disord, 2010. 25(3): p. 300–8.
Neumann, W.J., et al., Pallidal and thalamic neural oscillatory patterns in tourette’s syndrome. Ann Neurol, 2018. 84(4): p. 505–514.
Shute, J.B., et al., Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin, 2016. 12: p. 165–72.
Huebl, J., et al., Processing of emotional stimuli is reflected by modulations of beta band activity in the subgenual anterior cingulate cortex in patients with treatment resistant depression. Soc Cogn Affect Neurosci, 2016. 11(8): p. 1290–8.
Merkl, A., et al., Modulation of Beta-Band Activity in the Subgenual Anterior Cingulate Cortex during Emotional Empathy in Treatment-Resistant Depression. Cereb Cortex, 2015.
Neumann, W.J., et al., Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry, 2014. 19(11): p. 1186–92.
Bastin, J., et al., Changes of oscillatory activity in the subthalamic nucleus during obsessive-compulsive disorder symptoms: two case reports. Cortex, 2014. 60: p. 145–50.
Timmermann, L., et al., Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol, 2015. 14(7): p. 693–701.
Contarino, M.F., et al., Directional steering: A novel approach to deep brain stimulation. Neurology, 2014. 83(13): p. 1163–9.
Dembek, T.A., et al., Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord, 2017.
Pollo, C., et al., Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain, 2014. 137(Pt 7): p. 2015–26.
Reker, P., et al., Directional deep brain stimulation: A case of avoiding dysarthria with bipolar directional current steering. Parkinsonism Relat Disord, 2016. 31: p. 156–158.
Schupbach, W.M.M., et al., Directional leads for deep brain stimulation: Opportunities and challenges. Mov Disord, 2017.
Steigerwald, F., et al., Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord, 2016. 31(8): p. 1240–3.
Kühn, A.A. and J. Volkmann, Innovations in deep brain stimulation methodology. Movement Disorders, 2016.
Weiss, D., et al., Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain, 2013. 136(Pt 7): p. 2098–108.
Tinkhauser, G., et al., Directional local field potentials: A tool to optimize deep brain stimulation. Mov Disord, 2018. 33(1): p. 159–164.
Fernandez-Garcia, C., et al., Directional local field potential recordings for symptom-specific optimization of deep brain stimulation. Mov Disord, 2017. 32(4): p. 626–628.
Bour, L.J., et al., Directional Recording of Subthalamic Spectral Power Densities in Parkinson’s Disease and the Effect of Steering Deep Brain Stimulation. Brain Stimul, 2015. 8(4): p. 730–41.
Horn, A. and A.A. Kühn, Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage, 2015. 107: p. 127–35.
Horn, A., et al., Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol, 2017. 82(1): p. 67–78.
Caire, F., et al., A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson’s disease. Acta Neurochir (Wien), 2013. 155(9): p. 1647–54; discussion 1654.
Welter, M.L., et al., Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology, 2014. 82(15): p. 1352–61.
Petersen, M.V., et al., Using automated electrode localization to guide stimulation management in DBS. Ann Clin Transl Neurol, 2018. 5(7): p. 888–894.
Neumann, W.J., et al., Functional segregation of basal ganglia pathways in Parkinson’s disease. Brain, 2018. 141(9): p. 2655–2669.
Accolla, E.A., et al., Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain, 2016. 139(Pt 9): p. 2503–15.
Ramdhani, R.A., et al., Early Use of 60 Hz Frequency Subthalamic Stimulation in Parkinson’s Disease: A Case Series and Review. Neuromodulation, 2015. 18(8): p. 664–9.
Xie, T., et al., Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology, 2015. 84(4): p. 415–20.
Xie, T., U.J. Kang, and P. Warnke, Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 2012. 83(10): p. 1015–7.
Moreau, C., et al., STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology, 2008. 71(2): p. 80–4.
O'Suilleabhain, P.E., et al., Tremor response to polarity, voltage, pulsewidth and frequency of thalamic stimulation. Neurology, 2003. 60(5): p. 786–90.
Earhart, G.M., et al., Effects of Thalamic Stimulation Frequency on Intention and Postural Tremor. Exp Neurol, 2007. 208(2): p. 257–63.
Pedrosa, D.J., et al., Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp Neurol, 2013. 248: p. 205–12.
Fasano, A., et al., Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain, 2010. 133(Pt 12): p. 3635–48.
Groppa, S., et al., Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain, 2014. 137(Pt 1): p. 109–21.
Reich, M.M., et al., Progressive gait ataxia following deep brain stimulation for essential tremor: adverse effect or lack of efficacy? Brain, 2016. 139(11): p. 2948–2956.
Moldovan, A.S., et al., Less is more - Pulse width dependent therapeutic window in deep brain stimulation for essential tremor. Brain Stimul, 2018.
Reich, M.M., et al., Short pulse width widens the therapeutic window of subthalamic neurostimulation. Ann Clin Transl Neurol, 2015. 2(4): p. 427–32.
Steigerwald, F., et al., Pulse duration settings in subthalamic stimulation for Parkinson’s disease. Mov Disord, 2017.
Canessa, A., et al., Striatal Dopaminergic Innervation Regulates Subthalamic Beta-Oscillations and Cortical-Subcortical Coupling during Movements: Preliminary Evidence in Subjects with Parkinson’s Disease. Front Hum Neurosci, 2016. 10: p. 611.
Abbasi, O., et al., Unilateral deep brain stimulation suppresses alpha and beta oscillations in sensorimotor cortices. Neuroimage, 2018. 174: p. 201–207.
Arlotti, M., et al., Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology, 2018. 90(11): p. e971-e976.
Swann, N.C., et al., Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J Neurosurg, 2018. 128(2): p. 605–616.
Swann, N.C., et al., Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng, 2018. 15(4): p. 046006.
Weiss, D., et al., Subthalamic stimulation modulates cortical motor network activity and synchronization in Parkinson’s disease. Brain, 2015. 138(Pt 3): p. 679–93.
Whitmer, D., et al., High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci, 2012. 6: p. 155.
Wingeier, B., et al., Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson’s disease. Exp Neurol, 2006. 197(1): p. 244–51.
Eusebio, A., H. Cagnan, and P. Brown, Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front Integr Neurosci, 2012. 6: p. 47.
Fries, P., Rhythms for Cognition: Communication through Coherence. Neuron, 2015. 88(1): p. 220–35.
Malekmohammadi, M., et al., Pallidal stimulation in Parkinson disease differentially modulates local and network beta activity. J Neural Eng, 2018. 15(5): p. 056016.
Rosenbaum, R., et al., Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis, 2014. 62: p. 86–99.
Milosevic, L., et al., Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain, 2018. 141(7): p. 2142–2155.
Rosin, B., et al., Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron, 2011. 72(2): p. 370–84.
Malekmohammadi, M., et al., Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease. Mov Disord, 2016. 31(3): p. 426–8.
Pina-Fuentes, D., et al., Adaptive DBS in a Parkinson’s patient with chronically implanted DBS: A proof of principle. Mov Disord, 2017. 32(8): p. 1253–1254.
Rosa, M., et al., Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov Disord, 2017. 32(4): p. 628–629.
Ryapolova-Webb, E., et al., Chronic cortical and electromyographic recordings from a fully implantable device: preclinical experience in a nonhuman primate. J Neural Eng, 2014. 11(1): p. 016009.
Hebb, A.O., et al., Creating the feedback loop: closed-loop neurostimulation. Neurosurg Clin N Am, 2014. 25(1): p. 187–204.
Herron, J.A., et al., Cortical Brain-Computer Interface for Closed-Loop Deep Brain Stimulation. IEEE Trans Neural Syst Rehabil Eng, 2017. 25(11): p. 2180–2187.
Little, S., et al., Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 2015.
Little, S., et al., Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol, 2013.
Rosa, M., et al., Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord, 2015. 30(7): p. 1003–5.
Little, S., et al., Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry, 2016.
Swann, N.C., et al., Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci, 2016. 36(24): p. 6445–58.
Pina-Fuentes, D., et al., The characteristics of pallidal low-frequency and beta bursts could help implementing adaptive brain stimulation in the parkinsonian and dystonic internal globus pallidus. Neurobiol Dis, 2019. 121: p. 47–57.
Torkamani, A., et al., High-Definition Medicine. Cell, 2017. 170(5): p. 828–843.
Panov, F., et al., Intraoperative electrocorticography for physiological research in movement disorders: principles and experience in 200 cases. J Neurosurg, 2017. 126(1): p. 122–131.
Tsuboi, T., et al., Early detection of speech and voice disorders in Parkinson’s disease patients treated with subthalamic nucleus deep brain stimulation: a 1-year follow-up study. J Neural Transm (Vienna), 2017. 124(12): p. 1547–1556.
Shah, S.A., H. Tan, and P. Brown, Continuous Force Decoding from Deep Brain Local Field Potentials for Brain Computer Interfacing. Int IEEE EMBS Conf Neural Eng, 2017. 2017: p. 371–374.
Tan, H., et al., Decoding gripping force based on local field potentials recorded from subthalamic nucleus in humans. Elife, 2016. 5.
Flint, R.D., et al., Extracting kinetic information from human motor cortical signals. Neuroimage, 2014. 101: p. 695–703.
Islam, M.S., K.A. Mamun, and H. Deng, Decoding of Human Movements Based on Deep Brain Local Field Potentials Using Ensemble Neural Networks. Comput Intell Neurosci, 2017. 2017.
Mamun, K.A., et al., Movement decoding using neural synchronization and inter-hemispheric connectivity from deep brain local field potentials. J Neural Eng, 2015. 12(5): p. 056011.
Gers, F.A. and E. Schmidhuber, LSTM recurrent networks learn simple context-free and context-sensitive languages. IEEE Trans Neural Netw, 2001. 12(6): p. 1333–40.
Gers, F.A., J. Schmidhuber, and F. Cummins, Learning to forget: continual prediction with LSTM. Neural Comput, 2000. 12(10): p. 2451–71.
Xie, Z., O. Schwartz, and A. Prasad, Decoding of finger trajectory from ECoG using Deep Learning. J Neural Eng, 2017.
Alexander, G.E., M.R. DeLong, and P.L. Strick, Parallel organization of functionally segregated circuits linking basal ganglia. Annu Rev Neurosci, 1986. 9: p. 357–81.
Bostan, A.C., R.P. Dum, and P.L. Strick, The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A, 2010. 107(18): p. 8452–6.
Bostan, A.C. and P.L. Strick, The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci, 2018. 19(6): p. 338–350.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This review article was supported through the joint funding initiative of the United States National Institutes of Health (NIH 1 R01 NS110424) and the German Ministry for Education and Research (BMBF) “Collaborative Research in Computational Neuroscience (CRCNS)” to all authors of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 537 kb)
Rights and permissions
About this article
Cite this article
Neumann, WJ., Turner, R.S., Blankertz, B. et al. Toward Electrophysiology-Based Intelligent Adaptive Deep Brain Stimulation for Movement Disorders. Neurotherapeutics 16, 105–118 (2019). https://doi.org/10.1007/s13311-018-00705-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-018-00705-0