Abstract
The management of type 2 diabetes (T2D) often necessitates treatment intensification, and sometimes simplification to achieve glycaemic targets and mitigate complications. This expert opinion paper evaluates the use and positioning of the fixed-ratio combinations (FRCs) of basal insulin (BI) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in optimising T2D management. On the basis of the evidence presented and discussions, these FRCs offer a promising approach for both treatment intensification and simplification in people with suboptimal glucose control despite receiving various therapies. In treatment intensification, FRCs provide a synergistic effect by addressing multiple pathophysiological defects contributing to hyperglycaemia. These FRCs effectively control both fasting and postprandial glucose (PPG) excursions, offering significantly improved glycaemic control with a lower hypoglycaemia risk and weight neutrality compared to traditional or complex insulin regimens. Moreover, the reduced injection frequency (once daily) and flexibility in the dosing schedule (with any major meal of the day) help mitigate patient resistance to insulin initiation or titration. This further reduces treatment burden, facilitating treatment adherence and enhancing patient convenience. These key benefits of FRCs over complex insulin regimens play a crucial role in long-term glycaemic management and overall treatment outcomes. Hence, the timely use of FRCs in the treatment algorithm for people with T2D represents a valuable strategy for optimising glycaemic control, addressing treatment barriers and enhancing patient-reported outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In real-world settings, achieving optimal glycaemic targets remains challenging because of clinical inertia, lack of treatment adherence, disease burden and limited access to specialised healthcare professionals. |
Treatment intensification with complex insulin regimens can negatively affect both quality of life and treatment adherence as a result of side effects. |
Simplification using a fixed-ratio combination (FRC) of basal insulin and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) may help to decrease treatment complexity and reduce burden. |
Experts from South/Central Europe and Israel provided their recommendations on positioning of these FRCs in the treatment algorithm for individuals requiring insulin treatment intensification and/or simplification. |
All the experts unanimously agreed that FRCs can serve as a simplified treatment alternative to commonly used complex insulin regimens in individuals with T2D requiring treatment advancement and/or simplification. |
Introduction
Type 2 diabetes (T2D) is a major global public health concern contributing to approximately one million deaths annually from diabetes-related complications [1]. According to estimates by the International Diabetes Federation (IDF), 537 million people worldwide were living with diabetes in 2021, and this figure is projected to increase to 643 million by 2030 and 783 million by 2045 [2]. In Europe, the prevalence of diabetes is currently at 9.2%, with over 61.4 million people living with diabetes, which is expected to increase up to 10.4% by 2045 [2].
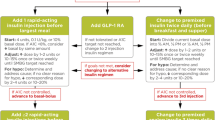
Treatment advancement to injectable therapies, such as insulin, often becomes imperative because of the progressive nature of the disease [3]. The updated guidelines from the American Diabetes Association (ADA)/the European Association for the Study of Diabetes (EASD) and the American Association for Clinical Endocrinology (AACE) suggest treatment intensification for individuals on oral antidiabetic drugs (OADs) with poorly controlled glycaemic levels by introducing injectable therapy, with an initial option being glucagon-like peptide 1 receptor agonists (GLP-1 RAs) or GIP (glucose-dependent insulinotropic polypeptide)/GLP-1 RA [4, 5]. Basal insulin (BI) or a combined injectable therapy could also be considered as the first therapy based on multiple factors, such as glycated haemoglobin (HbA1c) levels, other glycaemic parameters, lifestyle and patient characteristics [4, 5]. If required, further treatment intensification approaches include (a) adding GLP-1 RA or BI as a second injectable or switching to a fixed-ratio combination (FRC) of BI and GLP-1 RA; (b) switching to BI plus prandial insulin at mealtimes or a full basal-bolus insulin (BBI) regimen; or (c) switching to a premixed insulin regimen [4, 5].
Despite the availability of comprehensive guidelines and use of various therapeutics, glycaemic control remains suboptimal, leading to an increased risk of a wide range of diabetes-related complications, and thus creates substantial disease burden. Therefore, there is an urgent need for simple interventions and strategies to improve glucose control [6,7,8]. Poor glycaemic control is likely to be multifactorial and often arises from the delay in advancing therapy, often referred to as therapeutic inertia, affecting about 50% of the people with T2D [9, 10]. It is driven by a complex interplay between patient-, clinician- and healthcare system-related factors, and its improvement is pivotal in reducing the disease burden [11]. Treatment advancement from BI involves the sequential addition of a new glucose-lowering agent or a switch to a more complex insulin regimen, often associated with several challenges, such as frequent blood glucose monitoring, adapting multiple insulin doses to food intake and other daily activities, injection pain, and the risk of hypoglycaemia and weight gain. These factors lead to poor adherence and may also deter individuals and physicians from intensifying therapy [12].
To reduce the complexity and burden of diabetes therapy without compromising its effectiveness or safety, treatment simplification is advocated by some guidelines, especially for elderly, overtreated individuals or people with comorbidities [4, 5, 13]. These guidelines also recommend a proactive and patient-centric approach, involving regular monitoring, timely adjustment, shared decision-making and education [14, 15].
The FRCs containing BI and GLP-1 RA allow the administration of both agents in a single daily injection [16]. When compared to BI, FRCs provide simultaneous targeting of fasting plasma glucose (FPG) and postprandial glucose (PPG) [16], with no additional risk of hypoglycaemia [17,18,19,20] and weight neutrality or benefits [18,19,20]. This simplified approach may help address gaps in the care for BI treatment intensification, influencing patients’ willingness to intensify and potentially increasing treatment adherence [16]. In addition, the use of FRCs may lead to improved patient-reported outcomes (PROs), such as those related to treatment burden, diabetes management and mental health [21, 22].
Currently, there are two approved FRCs available for the management of T2D: a once-daily titratable FRC of BI glargine 100 U/mL (Gla-100) plus lixisenatide (iGlarLixi), and a once-daily titratable FRC of insulin degludec (IDeg) plus liraglutide (IDegLira). The efficacy and safety of these FRCs, henceforth referred to as iGlarLixi and IDegLira, have been established in the LixiLan and DUAL clinical development programmes, respectively [17,18,19,20, 23,24,25,26,27,28,29,30].
However, routine clinical practice involves heterogeneous real-life patient populations, and clinicians face numerous practical questions related to the use of this therapeutic class of FRC that cannot be answered current guidelines, summaries of product characteristics and recent clinical studies. Therefore, an advisory board meeting was conducted with experts from Central/South Europe and Israel to gain further insights into the use of this class for treatment intensification and simplification in the management of T2D.
Methods
The meeting was held at Hamburg, Germany on 4 October 2023. Experts from Czech Republic, Ukraine, Hungary, Romania, Poland and Israel were invited to discuss the following topics and share their opinions on the optimal use of FRCs in routine clinical practice for managing T2D during insulin treatment intensification and simplification:
-
To discuss the available clinical evidence on the use of FRC of BI and GLP-1 RA in insulin treatment intensification and simplification of a complex insulin regimen in people with T2D
-
To establish clear clinical criteria for people with T2D who could be suitable candidates for treatment with FRC of BI and GLP-1 RA in insulin treatment intensification and simplification of a complex insulin regimen
The available published evidence for the topics was gathered by the experts and discussed during the meeting. After the meeting, the minutes of the opinion and recommendations were circulated to resolve any disagreements among the experts. The recommendations summarised in this paper were based on a discussion on the latest diabetes management guidelines, data from the latest clinical studies and clinical practice of the expert panel.
Ethical Approval
This expert opinion manuscript is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Discussion
During the meeting, the experts agreed that glycaemic control in people with T2D is far from the desired target and that treatment inertia plays a major role in suboptimal glycaemic control. The experts aimed to formulate recommendations for optimal care of people with T2D based on clinical evidence and their real-time experience in clinical settings. The advantages and challenges associated with various insulin treatment intensification options available for T2D management are listed in Table 1.
Clinical Evidence Supporting the Use of FRC of BI and GLP-1 RA in Treatment Intensification
Owing to the distinct yet synergistic mode of action, the FRCs of BI and GLP-1 RA offer a promising combination therapy option for insulin-naïve people with T2D or individuals having uncontrolled or suboptimally controlled glycaemic levels on BI therapy. While BI lowers FPG levels by inhibiting the production of hepatic glucose, GLP-1 RAs stimulate insulin secretion and inhibit glucagon release. Furthermore, short-acting GLP-1 RAs significantly delay gastric emptying, a key mechanism contributing to PPG reduction [31].
The LixiLan randomised clinical trial (RCT) programme compared the safety and efficacy of iGlarLixi versus insulin glargine 100 U/mL (Gla-100) alone or lixisenatide alone in people with T2D uncontrolled on OADs (LixiLan-O) [20]; versus continued use of BI in people with T2D uncontrolled on BI with OADs (LixiLan-L) [19]; or versus continued use of GLP-1 RA therapy in people with T2D uncontrolled on GLP-1 RAs (LixiLan-G) [23]. The results from these trials showed that compared with other regimens, iGlarLixi provided greater reductions in HbA1c and improved glycaemic control, with a similar risk of hypoglycaemia and more favourable effect on body weight compared with Gla-100 and fewer gastrointestinal adverse events (AEs) compared with lixisenatide alone [19, 20, 23]. Furthermore, results from a network meta-analysis (NMA) of 17 studies comparing gastrointestinal AEs showed that fewer participants in the FRC group reported nausea and vomiting than those on single-agent GLP-1 RAs [32]. Another systematic review and NMA showed similar or improved efficacy and safety with iGlarLixi versus other intensification options, such as adding mealtime insulin or switching to premixed insulin [33]. Real-world studies echoed these findings, affirming that iGlarLixi leads to improved glycaemic control and reduction in body weight in people with T2D requiring treatment intensification from OADs or insulin therapy [34, 35].
The key studies in DUAL RCT programme evaluated the safety and efficacy of IDegLira in people with T2D inadequately controlled on OADs or IDeg plus OADs or GLP-1 RAs plus OADs; the DUAL I compared IDegLira with IDeg or liraglutide alone [17], DUAL II compared IDegLira with continued use of IDeg plus OADs [18] and DUAL III compared IDegLira with continued use of GLP-1 RAs plus OADs (DUAL III) [24]. The findings of these trials were similar to those observed in the LixiLan RCT programme. In brief, IDegLira resulted in greater reductions in HbA1c and improvement in glycaemic levels without weight gain as compared with other regimens. IDegLira was also associated with fewer gastrointestinal AEs than with liraglutide alone [17, 18]. Further, DUAL IV–IX studies investigated the use of IDegLira versus comparators, including continued OADs, Gla-100 or BBI therapy in people with T2D with varied treatment backgrounds [25,26,27,28,29,30]. In DUAL VII, IDegLira was non-inferior to BBI in HbA1c reduction and superior in body weight change, along with lower incidence of hypoglycaemia [28]. The post hoc analysis of the DUAL II Japan trial showed that switching from premixed insulin to IDegLira in individuals with uncontrolled glycaemic levels led to improved HbA1c, with underlying improvements in both FPG and PPG levels, and weight loss [36]. These results are also evident in real-world studies, where IDegLira resulted in greater HbA1c reductions, weight loss and a lower rate of hypoglycaemia versus conventional insulin therapy in people with T2D [37].
The experts agreed that the clinical data provide convincing data on the safety and efficacy of FRCs of BI and GLP-1 RA for use in people with T2D requiring treatment intensification beyond BI. These FRCs also demonstrate benefits over premixed insulin, BBI and GLP-1 RA therapy alone or with OADs.
Clinical Criteria for the Use of FRC of BI and GLP-1 RA in Intensification of BI in People with T2D
Optimal therapy in T2D management requires a balance between the benefits of glycaemic control and the risk of hypoglycaemia, which may improve adherence and quality of life (QoL) [38]. The current guidelines also emphasise early treatment intensification for optimal glycaemic control and avoiding therapeutic inertia [4]. The ADA/AACE guidelines recommend considering intensification of insulin therapy when BI dose is greater than 0.5 units/kg/day and HbA1c remains above the target level, either in combination with GLP-1 RAs or multiple doses of rapid-acting insulin analogues [4, 5]. The FRC of BI and GLP-1 RAs represents a synergic and complementary therapeutic strategy to achieve glucose control along with a low risk of hypoglycaemia as compared with intensified insulin regimens [39,40,41].
The experts agreed that defining the clinical criteria is pivotal for appropriate positioning of FRCs in the evolving therapeutic landscape. A clear and precise characterisation of people with T2D who need treatment advancement to achieve optimal glycaemic control would be beneficial for clinicians. This information helps them in making effective clinical decisions to avoid clinical inertia, and address adherence issues on insulin intensification. On the basis of the available literature and real-world experience so far, the experts recommend initiating FRCs for individuals with HbA1c levels above the target after BI therapy and requiring treatment intensification. The experts endorsed that individuals with HbA1c level greater than 9%, high PPG levels, obesity or a predisposition to high hypoglycaemia risk may potentially benefit from treatment intensification using FRCs. In addition, people with a longer diabetes duration requiring regimen modification or with a history of gastrointestinal AEs with previous GLP-1 RA treatment may also benefit from the use of FRCs. The treatment is also beneficial for people with a shorter duration of T2D and across all body mass index (BMI) subgroups. However, the experts also pointed out accessibility and cost as most important barriers, which may impact their use and position in the treatment landscape.
Clinical Evidence for the Use of FRC in Simplification of Complex Insulin Regimen in People with T2D
As a result of the advancing nature of T2D, most individuals eventually need to intensify their treatment to achieve optimal glycaemic control and prevent or delay diabetes-related complications. The treatment intensification typically involves the sequential addition of a new agent or switching to a more complex insulin regimen (e.g. multiple daily injection, MDI) [42]. Despite these complex insulin regimens, many people do not achieve their glycaemic goals [13, 43, 44]. Moreover, complex insulin regimen can lead to increased hypoglycaemia risk, weight gain and high treatment burden, resulting in poor treatment adherence and QoL [45, 46]. Growing evidence highlights the importance of simplifying complex treatment regimens in people with T2D [47, 48]. The updated ADA/EASD and AACE guidelines also advocate treatment simplification, particularly for insulin-based therapy. This approach may help decrease treatment complexity and burden leading to optimal glycaemic control and enhanced treatment adherence [4, 5, 9, 49].
Several studies have shown improved efficacy of FRCs of BI and GLP-1 RA when compared with other complex insulin regimens. iGlarLixi was found to be non-inferior and statistically superior in terms of change in HbA1c and body weight along with lower hypoglycaemia versus premixed insulin in the SoliMix trial [21]. In addition, greater improvement in overall treatment-related impact, satisfaction, treatment burden and diabetes management was observed with iGlarLixi versus premixed insulin in the subanalysis of the SoliMix trial [50]. In a post hoc propensity-score-matched analysis of two RCTs, compared with BBI, iGlarLixi demonstrated statistically significant reductions in HbA1c and a significantly lower rate of hypoglycaemia, along with weight loss [51]. Similarly, SoliSimplify, a real-world, retrospective, observational cohort study, further indicated that iGlarLixi provided similar glycaemic control without weight gain versus BBI therapy in people with T2D in a clinical practice setting [52]. Additionally, the subgroup analysis of the SPARTA Japan study showed that 6 months of treatment with iGlarLixi led to improved HbA1c and a decline in body weight with relatively less hypoglycaemia or gastrointestinal AEs across the subgroups of participants who had suboptimal glycaemic control on various prior treatment regimens, including MDI [53].
Likewise, the DUAL VII trial provided evidence supporting the rationale to use IDegLira over BBI therapy. Compared to BBI therapy, IDegLira was associated with a comparable reduction in HbA1c. However, it exhibited statistically significant lower rates of severe hypoglycaemia (p < 0.0001), reduction in mean body weight (p < 0.0001) and decrease in injection frequency [28]. Data from the DUAL II Japan trial showed that switching from premixed insulin to IDegLira was associated with a greater improvement in HbA1c and reduction in body weight [36]. Similarly, significant reductions were observed from baseline to 6 months in HbA1c, body weight and total daily insulin dose along with improvement in PROs in a real-world, prospective, observational study, which investigated the impact of switching from BBI to IDegLira [54]. European real-world studies showed that the transition from BBI therapy to IDegLira was associated with a significant reduction in HbA1c, body weight and total daily insulin dose (p < 0.001) and reduced hypoglycaemia incidence [55, 56].
The results from the BEYOND trial, an open-label RCT, conducted over 6 months, involved participants with T2D and inadequate glycaemic control. Switching from BBI to FRCs of BI and GLP-1 RAs (iGlarLixi and IDegLira) led to better HbA1c target achievement. Additionally, there was a reduction in insulin dose, the number of injections and the frequency of hypoglycaemia. These benefits persisted for up to 24 months in approximately 50% of the participants. At 6 months, there was no difference in HbA1c values between participants assigned to either IDegLira or iGlarLixi [57, 58].
The experts unanimously agreed that the available evidence supports the use of FRCs as an effective simplification strategy. They also concurred that most of the existing evidence is from retrospective analyses, and RCTs would be more beneficial and relevant in gaining additional insights into different clinical aspects.
Clinical Criteria for the Use of FRC of BI and GLP-1 RA in Simplification of Complex Insulin Regimen in People with T2D
Clinical criteria for selecting appropriate candidates for a simplification strategy with the FRC of BI and GLP-1 RA therapy depend on several factors, such as glycaemic targets, BMI, renal function, risk of hypoglycaemia and treatment preferences. Table 2 lists the potential characteristics of people with T2D who may benefit from the simplification of complex regimens using the FRC of BI and GLP-1 RA.
The experts highlighted that simplification is almost always successful in younger people with T2D with a short duration of diabetes, lower HbA1c, lower daily insulin dose and lack of microangiopathy. Furthermore, the experts discussed the profile of people with T2D who might benefit more from the simplification of complex insulin regimens. These people include:
-
Overtreated and overcontrolled individuals with T2D, defined as those receiving aggressive treatment and maintaining lower than recommended HbA1c levels (usually < 6.5%) and often experience frequent episodes of hypoglycaemia.
-
Overtreated and well-controlled individuals with T2D who have already achieved their individual HbA1c targets and may find that their current insulin regimen limits their QoL. Following a strict regimen could be inconvenient, or they may experience frequent episodes of hypoglycaemia.
-
Individuals with poorly controlled T2D, struggling with uncontrolled glucose levels and facing challenges in using MDIs due to inadequate titration, adherence issues (e.g. forgetting or skipping doses), technique problems (e.g. lipohypertrophy) and limitations in self-management, and may experience weight gain without achieving glycaemic goals. Increasing insulin doses for these individuals can lead to hypoglycaemia.
In all these instances, the therapeutic goal of transitioning from the complex insulin regimen to FRCs is to simplify the treatment plan, ensuring improved glycaemic control with reduced treatment burden and risk of associated AEs (such as hypoglycaemia and weight gain), thereby improving adherence and overall QoL. The experts agreed that the primary indication to switch from a complex insulin regimen to an FRC is inadequate glycaemic control or high HbA1c levels. The FRCs should be considered in instances where individualised HbA1c targets are not met despite using a BBI or premixed insulin regimen. FRCs may be preferred over BBI for individuals who desire regimen simplification, weight management and reduced risk of hypoglycaemia. The experts suggested utilising C-peptide levels in cases where severe insulin deficiency is suspected before considering the possibility of simplification using the FRC of BI and GLP-1 RAs.
In the interest of improving the efficacy of diabetes management during simplification, the experts highlighted some of the important steps to be considered:
-
Right after switching, the FRC dose should be uptitrated to attain individualised glycaemic levels.
-
Starting or uptitrating metformin to the maximally tolerated dose.
-
In individuals not on prior metformin, low-dose metformin (500 mg), preferably a metformin extended-release formulation, should be started 2–3 weeks after initiating the FRC to prevent gastrointestinal AEs.
-
Adding a sodium-glucose cotransporter 2 inhibitor (SGLT2i) to the FRC and metformin therapy improves efficacy and decreases cardiorenal risk.
-
Imparting patient and carer education to improve adherence.
Conclusions
The use of FRC of BI and GLP-1 RA in the management of T2D represents a simple and effective approach. It serves both as an option for treatment intensification in individuals receiving monotherapy with GLP-1 RA or BI and as a means of simplifying treatment for those with T2D on a complex insulin regimen. The strength of FRC of BI and GLP-1 RA lies in its efficacy in improving fasting and PPG levels, its weight-neutral or weight-reducing effect, low risk of hypoglycaemia, patient preferences, cost-effectiveness, and the flexibility to administer it before any major meal of the day.
In people with T2D receiving BI therapy, a constant uptitration of insulin doses may not always be effective in controlling blood glucose levels and can lead to side effects such as an elevated risk of hypoglycaemia and weight gain. Recognising that treatment simplicity plays a crucial role in long-term adherence, the ability to intensify treatment using the FRC of BI and GLP-1 RA through a single daily injection, along with reduced need for self-monitoring of blood glucose and lower total daily insulin doses, presents a convincing incentive.
The intensified insulin therapy has been often used as an ultimate approach to improve glucose levels in individuals with poor glucose control, especially for those with significant postprandial hyperglycaemia. Many of these individuals remain on this burdensome therapy for many years despite significant side effects, such as weight gain, hypoglycaemia and the need for frequent self-monitoring of blood glucose. In most cases, prandial insulin is administered empirically without proper dose adjustments as people with T2D often struggle with accurate carbohydrate calculations. Switching to the FRC of BI and GLP-1 RA has similar or better efficacy as intensified insulin therapy while leading to weight loss and a lower risk of hypoglycaemia. This approach can, in the long-term, also lead to a significant reduction in chronic diabetic complications owing to improved treatment adherence.
In summary, the FRC of BI and GLP-1 RA is considered a simple and effective therapeutic option for both intensification of BI and simplification of a complex insulin regimen. All experts agreed that all individuals with T2D should be assessed periodically for suitability of treatment simplification with the FRC of BI and GLP-1 RA.
Data Availability
The authors make themselves available to readers in case of any doubt, suggestion or question. Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–11.
International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: International Diabetes Federation; 2021.
Boeder S, Matamoros D, Mansy C. Practical guidance for healthcare providers on collaborating with people with type 2 diabetes: advancing treatment and initiating injectable therapy. Diabetes Ther. 2023;14(2):425–46.
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140–57.
Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29(5):305–40.
Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Diabetes Care. 2015;38(8):1615–21.
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
Skyler JS. Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clin Diabetes. 2004;22:162–6.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Rattelman CR, Ciemins EL, Stempniewicz N, Mocarski M, Ganguly R, Cuddeback JK. A retrospective analysis of therapeutic inertia in type 2 diabetes management across a diverse population of health care organizations in the USA. Diabetes Ther. 2021;12(2):581–94.
Gabbay RA, Kendall D, Beebe C, et al. Addressing therapeutic inertia in 2020 and beyond: a 3-year initiative of the American Diabetes Association. Clin Diabetes. 2020;38(4):371–81.
Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–5.
Jude EB, Malecki MT, Gomez Huelgas R, et al. Expert panel guidance and narrative review of treatment simplification of complex insulin regimens to improve outcomes in type 2 diabetes. Diabetes Ther. 2022;13(4):619–34.
Karam SL, Dendy J, Polu S, Blonde L. Overview of therapeutic inertia in diabetes: prevalence, causes, and consequences. Diabetes Spectr. 2020;33(1):8–15.
Zhu NA, Harris SB. Therapeutic inertia in people with type 2 diabetes in primary care: a challenge that just won’t go away. Diabetes Spectr. 2020;33(1):44–9.
Blonde L, Anderson JE, Chava P, Dendy JA. Rationale for a titratable fixed-ratio co-formulation of a basal insulin analog and a glucagon-like peptide 1 receptor agonist in patients with type 2 diabetes. Curr Med Res Opin. 2019;35(5):793–804.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885–93.
Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926–33.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972–80.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–35.
Rosenstock J, Emral R, Sauque-Reyna L, et al. Advancing therapy in suboptimally controlled basal insulin-treated type 2 diabetes: clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care. 2021;44(10):2361–70.
Miller E, Doshi A, Gron R, et al. IDegLira improves patient-reported outcomes while using a simple regimen with fewer injections and dose adjustments compared with basal-bolus therapy. Diabetes Obes Metab. 2019;21(12):2643–50.
Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G randomized clinical trial. Diabetes Care. 2019;42(11):2108–16.
Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8(1):101–14.
Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naive people with type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34(2):189–96.
Lingvay I, Perez Manghi F, Garcia-Hernandez P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315(9):898–907.
Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab. 2017;19(6):858–65.
Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009–16.
Aroda VR, Gonzalez-Galvez G, Gron R, et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open-label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):596–605.
Philis-Tsimikas A, Billings LK, Busch R, et al. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add-on to sodium-glucose co-transporter-2 inhibitor therapy: a randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(6):1399–408.
Perreault L, Rodbard H, Valentine V, Johnson E. Optimizing fixed-ratio combination therapy in type 2 diabetes. Adv Ther. 2019;36(2):265–77.
Rayner CK, Wu T, Aroda VR, et al. Gastrointestinal adverse events with insulin glargine/lixisenatide fixed-ratio combination versus glucagon-like peptide-1 receptor agonists in people with type 2 diabetes mellitus: a network meta-analysis. Diabetes Obes Metab. 2021;23(1):136–46.
Home P, Blonde L, Kalra S, et al. Insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi) compared with premix or addition of meal-time insulin to basal insulin in people with type 2 diabetes: a systematic review and Bayesian network meta-analysis. Diabetes Obes Metab. 2020;22(11):2179–88.
Dekic D, Bojic M, Janez A, et al. Effectiveness and safety of iGlarLixi in people with type 2 diabetes in Adriatic Region countries: ENSURE-ADR, a real-world study. Diabetes Ther. 2023;14(7):1217–29.
Hassanein M, Malek R, Shaltout I, et al. Real-world safety and effectiveness of iGlarLixi in people with type 2 diabetes who fast during Ramadan: the SoliRam observational study. Diabetes Metab Syndr. 2023;17(2): 102707.
Watada H, Ross Agner BF, Doshi A, Bardtrum L, Ranthe MF, Billings LK. IDegLira improves glycemic control in Japanese patients with uncontrolled type 2 diabetes on premixed insulin therapy. Diabetes Ther. 2020;11(1):331–9.
Szepkuti S, Bandur S, Kovacs G, et al. Real-world effectiveness of IDegLira compared with intensified conventional insulin therapy in adults with type 2 diabetes: a retrospective cohort study. BMC Endocr Disord. 2022;22(1):229.
Pogach L, Aron D. Balancing hypoglycemia and glycemic control: a public health approach for insulin safety. JAMA. 2010;303(20):2076–7.
Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017;40(4):614–24.
Maiorino MI, Chiodini P, Bellastella G, et al. The good companions: insulin and glucagon-like peptide-1 receptor agonist in type 2 diabetes. A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2019;154:101–15.
Cimmaruta D, Maiorino MI, Scavone C, et al. Efficacy and safety of insulin-GLP-1 receptor agonists combination in type 2 diabetes mellitus: a systematic review. Expert Opin Drug Saf. 2016;15(Suppl 2):77–83.
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6.
Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–9.
Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–75.
Spain CV, Wright JJ, Hahn RM, Wivel A, Martin AA. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653-64.e1.
Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am Psychol. 2016;71(7):539–51.
Bohm AK, Schneider U, Aberle J, Stargardt T. Regimen simplification and medication adherence: fixed-dose versus loose-dose combination therapy for type 2 diabetes. PLoS One. 2021;16(5): e0250993.
Naing S, Ramesh G, Garcha J, Poliyedath A, Khandelwal S, Mills PK. Is the stepping-down approach a better option than multiple daily injections in obese patients with poorly controlled type 2 diabetes on advanced insulin therapy? Endocrinol Diabetes Metab. 2021;4(2): e00204.
Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2020 Executive Summary. Endocr Pract. 2020;26(1):107–39.
Polonsky WH, Giorgino F, Rosenstock J, et al. Improved patient-reported outcomes with iGlarLixi versus premix BIAsp 30 in people with type 2 diabetes in the SoliMix trial. Diabetes Obes Metab. 2022;24(12):2364–72.
Tabak AG, Anderson J, Aschner P, et al. Efficacy and safety of iGlarLixi, fixed-ratio combination of insulin glargine and lixisenatide, compared with basal-bolus regimen in patients with type 2 diabetes: propensity score matched analysis. Diabetes Ther. 2020;11(1):305–18.
McCrimmon RJ, Cheng AYY, Galstyan G, et al. iGlarLixi versus basal plus rapid-acting insulin in adults with type 2 diabetes advancing from basal insulin therapy: the SoliSimplify real-world study. Diabetes Obes Metab. 2023;25(1):68–77.
Miyoshi H, Matsuhisa M, Yabe D, Takahashi Y, Morimoto Y, Terauchi Y. Use of iGlarLixi for the management of type 2 diabetes in Japanese clinical practice: prior treatment subgroup analysis of the SPARTA Japan study. Diabetes Ther. 2023;14(4):671–89.
Persano M, Nollino L, Sambataro M, et al. Real-world study on the effectiveness and safety of basal insulin IDegLira in type 2 diabetic patients previously treated with multi-injective insulin therapy. Eur Rev Med Pharmacol Sci. 2021;25(2):923–31.
Price H, Bluher M, Prager R, et al. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20(4):954–62.
Taybani ZJ, Botyik B, Gyimesi A, Katko M, Varkonyi T. One-year safety and efficacy results of insulin treatment simplification with IDegLira in type 2 diabetes. Endocrinol Diabetes Metab. 2023;6(1): e390.
Giugliano D, Longo M, Caruso P, et al. Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a GLP-1RA or to basal insulin plus an SGLT2 inhibitor: BEYOND, a randomized, pragmatic trial. Diabetes Care. 2021;44(6):1353–60.
Giugliano D, Longo M, Scappaticcio L, et al. BEYOND 2 years: durability of metabolic benefits by simplification of complex insulin regimens in type 2 diabetes. Endocrine. 2024;83(2):399–404.
Medical Writing
Manuscript writing assistance was provided by Sneha Sinha and Preeti Agarwal, both employees of Sanofi.
Funding
This expert meeting was funded by Sanofi. The authors received no payment from Sanofi related to the development of this publication. The Rapid Service Fee to journal was funded by Sanofi. Sanofi had the opportunity to review the publication.
Author information
Authors and Affiliations
Contributions
All authors were involved in writing and editing various drafts of the manuscript. The authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for the authorship for this article, take the responsibility for the integrity of the work and have given their approval for this version to be published. Martin Haluzik: was chairman of the expert meeting, provided the draft of the manuscript based on expert meeting discussion, leading the manuscript preparation, approving the final draft of the manuscript. Zoltan Taybani: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript. Aleksandra Araszkiewicz: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript. Anca Cerghizan: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript. Boris Mankovsky: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript. Agbaria Zuhdi: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript. Maciej Malecki: contribution to forming the expert opinion, participation in the manuscript preparation, approving the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Martin Haluzik has been an advisory panel member of Novo Nordisk, Eli Lilly, Sanofi, Merck, Pfizer, Astra Zeneca and Berlin-Chemie; has received consulting fees and grants/research support from Sanofi; has received the speakers honoraria for speakers bureau from Sanofi, Novo Nordisk, Eli Lilly, Sanofi, Pfizer, AstraZeneca and Berlin-Chemie. Zoltan Taybani has been an advisory panel member of Boehringer Ingelheim; has been a consultant for AstraZeneca and Novo Nordisk; has received the speakers honoraria for speakers bureau from Sanofi. Aleksandra Araszkiewicz has received lecture fees or advisory board fees from Eli Lilly, Novo Nordisk and Sanofi. Anca Cerghizan has been an advisory panel member of Sanofi, AstraZeneca, Novo Nordisk, Boehringer Ingelheim and Medtronic; has received educational grants from Worwag, Eli Lilly, Novo Nordisk, Sanofi, Servier, Berlin-Chemie, MSD, Terapia, AstraZeneca and Amgen; has participated in clinical studies conducted by Sanofi, MSD, Johnson & Johnson, Berlin-Chemie Menarini, Roche Pharma Holding, AstraZeneca and Novo Nordisk; has received speakers honoraria for speakers bureau from Sanofi, Novo Nordisk, Eli Lilly, MSD, Boehringer Ingelheim, AstraZeneca, BMS, Terapia, Servier, KRKA, Milan, Egis, Medtronic, Medochemie and Gedeon. Boris Mankovsky has received the speakers honoraria for speakers bureau from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi, Servier and Bayer; has been a member of advisory board of Sanofi, Bayer, Worwag and Boehringer Ingelheim. Agbaria Zuhdi has received speaker honoraria for advisory board meeting from Sanofi. Maciej Malecki has acted as a consultant or speaker for AstraZeneca, Bayer, Novo Nordisk, Sanofi-Aventis, Lilly, Merck, Boehringer Ingelheim, Mundipharma, Abbott, Dexcom, and Medtronic; has also participated in the National Center for Research and Development CRACoV-HHS project.
Ethical Approval
This expert opinion manuscript is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Haluzik, M., Taybani, Z., Araszkiewicz, A. et al. Expert Opinion on Optimising Type 2 Diabetes Treatment Using Fixed-Ratio Combination of Basal Insulin and GLP-1 RA for Treatment Intensification and Simplification. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01610-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01610-w