Abstract
Introduction
This systematic review aimed to summarize the existing evidence from published randomized controlled trials (RCTs) on the impact of sodium–glucose cotransporter (SGLT) inhibitors on albuminuria levels and renal function in patients with type 1 diabetes mellitus (T1D).
Methods
The literature search was performed through Medline (via PubMed), Cochrane Library, and Scopus until November 11, 2023. Double-independent study selection, data extraction, and quality assessment were performed. Evidence was pooled with three-level mixed-effects meta-analysis.
Results
In total, 5221 participants with T1D among 11 RCTs were analyzed. All RCTs had low risk of bias according to the Cochrane Collaboration tool (RoB 2). SGLT inhibitors were associated with a significantly greater reduction in urine albumin-to-creatinine ratio (UACR) compared to controls (MD = − 23.13%; 95% CI = [− 33.69, − 12.57]; P < 0.001; level of evidence high). On the basis of subgroup analysis, this effect was consistent across all available SGLT inhibitors, irrespective of the dosage. Finally, a neutral class effect was observed on the estimated glomerular filtration rate (eGFR, MD = − 1.03 mL/min/1.73 m2; 95% CI = [− 2.26, 0.19]; P = 0.1; level of evidence moderate). Only empagliflozin was associated with a significant reduction in eGFR compared to placebo (MD = − 2.23 mL/min/1.73 m2; 95% CI = [− 3.62, − 0.84]; P = 0.002).
Conclusion
Our findings suggest that adjunctive therapy with SGLT inhibitors results in a significant reduction in albuminuria, while their use is associated with a neutral effect on creatinine clearance, as a measure of renal function. Future renal outcome trials are needed to assess SGLT inhibitors’ role in the pharmacological armamentarium against diabetic nephropathy in T1D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic kidney disease (CKD) affects 30–40% of individuals with type 1 diabetes (T1D). |
Regression of albuminuria, indicated by a reduction in urine albumin-to-creatinine ratio (UACR), has been shown to mitigate the risk of diabetic nephropathy and cardiovascular complications. |
The present meta-analysis suggests that adjunctive therapy with sodium–glucose cotransporter (SGLT) inhibitors is associated with a significantly greater reduction in the UACR and exhibits a neutral class effect on the estimated glomerular filtration rate. |
Future renal outcome trials are warranted to assess SGLT inhibitors’ role in the pharmacological armamentarium against diabetic nephropathy in T1D. |
Introduction
Chronic kidney disease (CKD) affects 30–40% of individuals with type 1 diabetes (T1D), representing an additional cardiovascular risk factor [1]. According to evidence from the Finnish Diabetic Nephropathy (FinnDiane) study, CKD increases the mortality rate by 3.6-fold in patients with T1D compared to the general population [2]. Indeed, the presence of microalbuminuria, macroalbuminuria, and end-stage kidney disease (ESKD) was associated with 2.8, 9.2, and 18.3 times higher standardized mortality ratio, respectively [2].
Hyperglycemia, high blood pressure, and albuminuria are the most important risk factors for CKD progression among individuals with T1D [1]. The incidence rate of severe albuminuria in subjects diagnosed with T1D from the 1970s to the 1980s decreased almost by 50%, coinciding with the development of renin–angiotensin system blockers [3]. The absence of subsequent positive development underscores the urgent need for novel renoprotective therapeutic strategies in T1D [1].
Sodium–glucose cotransporter (SGLT) inhibitors constitute a novel class of glucose-lowering drugs with a unique mechanism of action. Beyond their antihyperglycemic effects, which is independent of endogenous insulin secretion, these inhibitors also mitigate the risk of CKD progression, irrespective of the presence of type 2 diabetes mellitus (T2D) [4]. However, trials confirming those renoprotective properties of SGLT inhibitors did not include individuals with T1D.
Hitherto, several randomized controlled trials (RCTs) have assessed the safety and efficacy of SGLT inhibitors as adjunctive therapy in T1D. Herein, we present the first comprehensive systematic review and meta-analysis that summarizes the effect of SGLT inhibitors on renal function indices among individuals with T1D.
Methods
This study adhered to the principles of the Cochrane Handbook for systematic reviews [5] and the reporting conformed to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) 2020 guidelines [6]. The protocol was prospectively registered on the Open Science Framework (https://doi.org/10.17605/OSF.IO/KJ5YS) and was adhered to without deviations. As this is a meta-analysis of published RCTs, no ethical approval was required.
Search Strategy
Two independent researchers conducted a comprehensive literature search on MEDLINE (via PubMed), Scopus, and the Cochrane Database of Systematic Reviews, spanning from the inception of the databases to November 11, 2023. During the search process, no language restrictions were imposed. The search queries employed basic terms in both free text and Medical Subject Headings format, including “sglt inhibitors,” “empagliflozin,” “dapagliflozin,” “sotagliflozin,” “ipragliflozin,” “type 1 diabetes mellitus,” and “randomized controlled trial.” Additionally, searches were expanded by manual exploration of the Epistemonikos database and Google Scholar search engine. We also utilized the citationchaser R package for backward and forward citation chasing [7]. The detailed search strategy can be found in Supplemental Tables 1–3.
Eligibility Criteria
Inclusion Criteria
Eligible studies were phase 2 or above RCTs investigating the effect of SGLT2 inhibitors on albuminuria levels and estimated glomerular filtration rate (eGFR), as adjunctive to insulin in adults (> 18 years) with established T1D, compared to placebo.
Exclusion Criteria
Studies with the following characteristics were excluded:
-
Trials enrolling pediatric population
-
Short duration (less than 4 weeks)
-
Including individuals with T2D or healthy controls
-
Case reports/case series, narrative reviews
-
Editorials, letters, commentaries, expert opinions, clinical practice guidelines, conference abstracts, dissertations, protocols
-
Including animals
-
Observational studies
-
Not retrievable full text
Study Selection
Firstly, the records obtained through the described search strategy underwent independent screening of titles and abstracts by two authors. To improve the sensitivity of our study selection process, any disagreements during this stage did not result in exclusions. Subsequently, the same authors individually performed in-depth assessments of the full-text content. Any disagreements were addressed through discussions or by involving a third author with additional expertise in the field. The online software Abstrackr [8] was used for screening in the initial phase. Mendeley was used for full-text screening and reference management.
Data Extraction
We initially created a data extraction form, which was then tested on a subset of three studies as part of a pilot extraction process. After discussions, training, and calibration exercises, we established a final, standardized data extraction form. This process was carried out independently and in duplicate, and any inconsistencies were resolved through discussions or by engaging a third author with more expertise in the field, if necessary. In case of missing evidence or discrepant data we contacted the authors of the primary studies. For each study, we extracted data pertaining to sample size, key clinical and demographic characteristics, as well as changes in the outcomes of interest.
Risk of Bias (RoB) Assessment
Two authors independently assessed the risk of bias in the included studies, taking into account all predetermined domains in the revised version of the Cochrane Collaboration tool (RoB 2) [9]. Any discrepancies were resolved through discussion or by including a third author with higher expertise.
Data Analysis
R Statistical Software (v. 4.2) was employed for all analyses. Categorical variables were represented as absolute frequencies with percentages (%), while continuous variables were presented as mean with standard deviation (SD) if normally distributed; otherwise, as median with interquartile range (IQR). To assess differences between intervention and control groups for continuous outcomes, mean difference (MD) was utilized.
Given that each RCT reported multiple effect sizes corresponding to various doses of SGLT inhibitors, three-level meta-analytic mixed-effects models were applied. These models included a random effect for each study and another random effect for each experiment nested within a study. This approach facilitated the appropriate handling of correlated effect sizes within studies and accounted for potential dependencies among them.
The difference in albumin-to-creatinine ratio (UACR) between intervention and control groups was expressed as a percentage (%), while the difference in estimated eGFR was expressed as mL/min/1.73 m2. Subgroup analyses were conducted on the basis of the different available SGLT inhibitors and different dosages. For summary treatment effect estimates, a significance threshold of a two-tailed P value less than 0.05 was used. Visual summaries of all results were presented using forest plots.
The I2 value, which quantifies inconsistency across studies, was employed to estimate the percentage of total variability attributed to between-study heterogeneity. This was formally tested using Cochran’s Q test. I2 values, ranging from zero to one, indicate the extent of heterogeneity between studies, with values closer to one signifying greater heterogeneity. Roughly, cutoff values of 25%, 50%, and 75% denote low, moderate, and high heterogeneity, respectively. Exploration of small study effects, including an examination of publication bias, was visually represented using contour-enhanced funnel plots depicting effect size versus standard error. Formal testing was conducted using Egger’s test.
Certainty of Evidence
Certainty assessments for each outcome were performed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) checklist [10].
Results
Study Selection and Characteristics
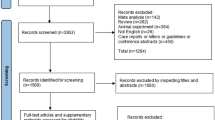
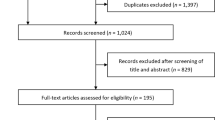
The flow diagram of the study selection process is concisely presented in Supplemental Fig. 1. Initially, the 5961 identified records underwent screening based on their title and abstract. Out of these, 5943 records were excluded and 18 records were chosen for a comprehensive full-text evaluation. Eight studies, including a total of 11 RCTs (5221 participants), met the inclusion criteria [11,12,13,14,15,16,17,18]. A list of excluded studies with reasoning is provided in Supplemental Table 4.
Τhe main characteristics of included trials are summarized in Table 1. In total, 3318 patients received adjunctive treatment with SGLT inhibitors. Among them, 1791 (54%) were treated with sotagliflozin, 1231 (37.1%) with empagliflozin, 181 (5.5%) with dapagliflozin, and 115 (3.5%) with ipragliflozin. The control group comprised 1903 participants who received a placebo in addition to standard insulin therapy. The median diabetes duration was 21.7 years, whereas the mean participants’ age ranged from 21.7 to 55 years. The participants had a mean baseline glycated hemoglobin (HbA1c) above the target range, between 7.4% and 9.9% and the median treatment duration was 24 weeks.
According to the RoB 2 assessment tool, the risk of bias, both overall and in specific domains, was consistently low across all the included RCTs (Supplemental Fig. 2).
Urine Albumin-to-Creatinine Ratio
Figure 1 summarizes the results of the main and subgroup analyses. Overall, the 2420 patients with T1D and a high baseline UACR (≥ 30 mg/g) treated with SGLT inhibitors experienced a significantly greater reduction in UACR compared to controls (MD = − 23.13%; 95% CI = [− 33.69, − 12.57]; P < 0.001; I2 = 0%; P = 0.9; Fig. 1A). This effect was consistent across all types of SGLT inhibitors. The subgroup analysis based on the different dosages of SGLT inhibitors confirmed that the benefit in terms of UACR reduction was significant irrespective of the dosage (MD = − 18.15%; 95% CI = [− 33.89, − 2.42]; P < 0.001; I2 = 0%; P = 0.83, for lower dose and MD = − 27.12%; 95% CI = [− 41.44, − 12.97]; P < 0.001; I2 = 0%; P = 0.75, for higher dose; P for subgroup differences = 0.4; Fig. 1B). No evidence of small-study effects was identified on the basis of the symmetrical funnel plot and Egger’s test (Supplementary Fig. 3).
Estimated Glomerular Filtration Rate
Considering the class effect of SGLT inhibitors on eGFR, a non-significant change was documented compared to control group (MD = − 1.03 mL/min/1.73 m2; 95% CI = [− 2.26, 0.19]; P = 0.1; I2 = 58%; P < 0.01; Fig. 2). Empagliflozin resulted in a significantly greater reduction in eGFR compared to placebo (MD = − 2.23 mL/min/1.73 m2; 95% CI = [− 3.62, − 0.84]; P = 0.002; I2 = 64%; P = 0.02). No evidence of small-study effects was observed on the basis of the symmetrical funnel plot and Egger’s test (Supplementary Fig. 4).
GRADE Assessment
The level of evidence was estimated as high for the effect of SGLT inhibitors on UACR, while it was deemed moderate for the effect on eGFR due to concerns related to inconsistency (Supplemental Table 5).
Discussion
This is the first relevant systematic review and meta-analysis assessing the effect of SGLT inhibitors on albuminuria levels in individuals with T1D. Our meta-analysis demonstrated that adjunctive therapy with SGLT inhibitors resulted in a significant reduction in UACR by 23.13%, irrespective of the dosage, and a neutral effect on eGFR in subjects with T1D. With the exception of empagliflozin, which was associated with a significantly greater reduction in eGFR, these findings could be regarded as a class effect. The present results expand existing knowledge on the pleiotropic beneficial effects of SGLT inhibitors beyond glycemic control, highlighting their role in reducing albuminuria and preserving renal function over the long term in patients with T1D.
In individuals with T1D, regression of albuminuria, indicated by a reduction in UACR, has been shown to mitigate the risk of diabetic nephropathy and cardiovascular complications [2]. The resultant risk levels closely align with those observed in individuals without albuminuria [2]. The renoprotective benefits of SGLT inhibitors in terms of reduction in UACR and eGFR stabilization have been previously documented in individuals with T2D across different stages of kidney disease [19,20,21]. Of note, as our findings indicate that the magnitude of UACR reduction in T1D was generally consistent with what has been reported in the T2D population [19,20,21].
A former meta-analysis of RCTs including 5397 individuals with T1D showed that adjunctive therapy with SGLT inhibitors resulted in a significant decrease in eGFR compared with placebo (WMD − 1.87; 95% CI = [− 2.58 to − 1.15]) [22]. In the subgroup analysis, SGLT inhibitors at both high and moderate dosages showed an equivalent effect on eGFR levels. Another meta-analysis including 4215 participants with T1D revealed that SGLT inhibitors significantly reduced eGFR levels when compared with placebo (WMD − 0.67; 95% CI [− 0.71 to − 0.63]). On the basis of the subgroup analysis, both 6-month and 12-month treatment with SGLT inhibitors led to a significant decrease in eGFR. Furthermore, this adverse effect increased with the longer duration of SGLT inhibitor treatment, as a significant difference was observed between the 6-month and 12-month treatment subgroups [23].
On the other hand, in a recent robust real-world study that included 1882 propensity-matched patients treated with glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and 992 patients treated with SGLT inhibitors for at least 6 months, SGLT inhibitor therapy led to the preservation of eGFR over a 5-year period, even in those with established CKD, while GLP-1 RAs therapy failed to achieve the same result [24]. At the same time, individuals treated with SGLT inhibitors were 51% less likely to develop CKD compared to those treated with GLP-1 RAs [24]. Finally, in a retrospective, multicenter cohort study investigating the effects of 24-month dapagliflozin treatment on renal function among 295 T1D individuals, dapagliflozin users experienced a significantly smaller decrease in eGFR levels than non-users [25]. In addition, the median change in UACR differed significantly between dapagliflozin users and non-users [25].
Strengths and Limitations
Although the current study was conducted rigorously, adhering to pertinent methodological and reporting guidelines, and following a preregistered protocol, it is important to acknowledge some limitations. Firstly, the between-study heterogeneity considering the eGFR was significant, which could be partially attributed to the variability of sample size, length of follow-up, and titration algorithms. Moreover, despite the beneficial, documented effect on UACR levels in individuals with high baseline UACR levels, the lack of data prevented the investigation of patients without baseline albuminuria. Finally, as a result of the limited number of primary studies, it was not possible to address potential effect modification using meta-regression analysis. Recent meta-analyses have indeed reported a beneficial effect of SGLT inhibitors on glycemic control and blood pressure reduction [26, 27]. Hence, further studies could convincingly answer the ubiquitous question of whether the observed effect of SGLT inhibitor on renal markers is a sign of a true underlying pathophysiological link or a mere epiphenomenon.
Conclusions
SGLT inhibitors exert significant benefit in terms of albuminuria reduction and a non-significant effect on eGFR levels in patients with T1D, when added to standard insulin treatment. Future, large, well-designed, renal outcome trials are required to shed further light on their exact place in the treatment of patients with T1D, with emphasis on the prevention or delaying of CKD, which still represents a major complication in clinical practice affecting life and its quality for patients with T1D [25].
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Heerspink HJ, Cherney DZ, Groop P-H, et al. People with type 1 diabetes and chronic kidney disease urgently need new therapies: a call for action. Lancet Diabetes Endocrinol. 2023;11:536–40. https://doi.org/10.1016/S2213-8587(23)00168-7.
Groop P-H, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–8. https://doi.org/10.2337/db08-1543.
Jansson Sigfrids F, Groop P-H, Harjutsalo V. Incidence rate patterns, cumulative incidence, and time trends for moderate and severe albuminuria in individuals diagnosed with type 1 diabetes aged 0–14 years: a population-based retrospective cohort study. Lancet Diabetes Endocrinol. 2022;10:489–98. https://doi.org/10.1016/S2213-8587(22)00099-7.
Baigent C, Emberson J, Haynes R, et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–801. https://doi.org/10.1016/S0140-6736(22)02074-8.
Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Training. https://training.cochrane.org/handbook/current. Accessed Jan 17, 2023.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. https://doi.org/10.1136/BMJ.N71.
Haddaway NR, Grainger MJ, Gray CT. citationchaser: an R package and Shiny app for forward and backward citations chasing in academic searching; 2021. https://doi.org/10.5281/ZENODO.4543513.
Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. IHI’12—Proc 2nd ACM SIGHIT Int Heal Informatics Symp. 2012:819–23. https://doi.org/10.1145/2110363.2110464.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. https://doi.org/10.1136/BMJ.L4898.
GRADEpro. https://www.gradepro.org/. Accessed March 1, 2023.
Haidar A, Lovblom LE, Cardinez N, et al. Empagliflozin add-on therapy to closed-loop insulin delivery in type 1 diabetes: a 2 × 2 factorial randomized crossover trial. Nat Med. 2022;28:1269–76. https://doi.org/10.1038/s41591-022-01805-3.
Cherney DZI, Bjornstad P, Perkins BA, et al. Kidney effects of empagliflozin in people with type 1 diabetes. Clin J Am Soc Nephrol. 2021;16:1715–9. https://doi.org/10.2215/CJN.07700621.
Bode BW, Cengiz E, Wadwa RP, et al. Effects of sotagliflozin combined with intensive insulin therapy in young adults with poorly controlled type 1 diabetes: the JDRF sotagliflozin study. Diabetes Technol Ther. 2021;23:59–69. https://doi.org/10.1089/dia.2020.0079.
Groop P-H, Dandona P, Phillip M, et al. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8:845–54. https://doi.org/10.1016/S2213-8587(20)30280-1.
van Raalte DH, Bjornstad P, Persson F, et al. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care. 2019;42:1921–9. https://doi.org/10.2337/dc19-0937.
Kaku K, Isaka H, Sakatani T, Toyoshima J. Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double-blind, phase 3 trial. Diabetes Obes Metab. 2019;21:2284–93. https://doi.org/10.1111/dom.13807.
Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337–48. https://doi.org/10.1056/NEJMoa1708337.
Kuhadiya ND, Ghanim H, Mehta A, et al. Dapagliflozin as additional treatment to liraglutide and insulin in patients with type 1 diabetes. J Clin Endocrinol Metab. 2016;101:3506–15. https://doi.org/10.1210/jc.2016-1451.
Pollock C, Stefánsson B, Reyner D, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, pla. Lancet Diabetes Endocrinol. 2019;7:429–41. https://doi.org/10.1016/S2213-8587(19)30086-5.
Fioretto P, Del Prato S, Buse JB, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab. 2018;20:2532–40. https://doi.org/10.1111/dom.13413.
Yale J-F, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. https://doi.org/10.1111/dom.12090.
Li K, Xu G. Safety and efficacy of sodium glucose co-transporter 2 inhibitors combined with insulin in adults with type 1 diabetes: a meta-analysis of randomized controlled trials. J Diabetes. 2019;11:645–55. https://doi.org/10.1111/1753-0407.12890.
Huang Y, Jiang Z, Wei Y. Short- and medium-term efficacy of sodium glucose cotransporter 2 (SGLT-2) inhibitors for the treatment of type 1 diabetes: systematic review and meta-analysis. Endokrynol Pol. 2020;71:325–33. https://doi.org/10.5603/EP.a2020.0034.
Anson M, Zhao SS, Austin P, Ibarburu GH, Malik RA, Alam U. SGLT2i and GLP-1 RA therapy in type 1 diabetes and reno-vascular outcomes: a real-world study. Diabetologia. 2023;66:1869–81. https://doi.org/10.1007/s00125-023-05975-8.
Hironaka J, Okada H, Hamaguchi M, et al. Effects of dapagliflozin on renal function in type 1 diabetes patients in the real world: a retrospective multicenter study of the KAMOGAWA-A cohort. Diabetes Res Clin Pract. 2023;202: 110794. https://doi.org/10.1016/j.diabres.2023.110794.
Nan J, Wang D, Zhong R, et al. Sodium glucose cotransporter2 inhibitors for type 1 diabetes mellitus: a meta-analysis of randomized controlled trials. Prim Care Diabetes. 2023. https://doi.org/10.1016/j.pcd.2023.10.010.
Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the risk of ketoacidosis due to sodium–glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: a meta-analysis and meta-regression. PLoS Med. 2020;17:e1003461. https://doi.org/10.1371/journal.pmed.1003461.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Paschalis Karakasis: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Visualization, Project administration, Writing—original draft, Writing—review and editing. Djordje S. Popovic: Conceptualization, Methodology, Investigation, Project administration, Writing—original draft, Writing—review and editing. Dimitrios Patoulias: Investigation, Writing—review and editing. Theocharis Koufakis: Writing—review and editing. Nikolaos Papanas: Writing—review and editing. Nikolaos Fragakis: Validation, Writing—review and editing. Manfredi Rizzo: Conceptualization, Validation, Writing—review and editing, Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Djordje S. Popovic declares associations with Abbott, Alkaloid, Amicus, AstraZeneca, Boehringer-Ingelheim, Berlin-Chemie, Eli Lilly, Galenika, Krka, Merck, Novo Nordisk, PharmaSwiss, Sanofi-Aventis, Servier, Viatris, and Wörwag Pharma. Theocharis Koufakis has received honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Pharmaserve Lilly, and Novo Nordisk, for advisory boards from Novo Nordisk and Boehringer-Ingelheim, and has participated in sponsored studies by Eli Lilly and Novo Nordisk. Nikolaos Papanas has been an advisory board member of AstraZeneca, Boehringer-Ingelheim, MSD, Novo Nordisk, Pfizer, Takeda and TrigoCare International; has participated in sponsored studies by AstraZeneca, Eli Lilly, GSK, MSD, Novo Nordisk, Novartis and Sanofi-Aventis; has received honoraria as a speaker for AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Elpen, MSD, Mylan, Novo Nordisk, Pfizer, Sanofi-Aventis and Vianex; and has attended conferences sponsored by TrigoCare International, Eli Lilly, Galenica, Novo Nordisk, Pfizer and Sanofi-Aventis. Manfredi Rizzo is Editorial Board Member of Diabetes Therapy and has given lectures, received honoraria and research support, and participated in conferences, advisory boards, and clinical trials sponsored by many pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, Kowa, Eli Lilly, Meda, Mylan, Merck Sharp & Dohme, Novo Nordisk, Novartis, Roche Diagnostics, Sanofi, and Servier. None of the aforementioned pharmaceutical companies had any role in this article, which has been written independently, without any financial or professional help, and reflects only the opinion of the authors, without any role of the industry.
Ethical Approval
This study adhered to the principles of the Cochrane Handbook for systematic reviews [5] and the reporting conformed to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) 2020 guidelines [6]. The protocol was prospectively registered on the Open Science Framework (https://doi.org/10.17605/OSF.IO/KJ5YS) and was adhered to without deviations. As this is a meta-analysis of published RCTs, no ethical approval was required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Karakasis, P., Popovic, D.S., Patoulias, D. et al. The Effect of Sodium–Glucose Cotransporter Inhibitors on Renal Function as Adjunctive to Insulin in Adults with Type 1 Diabetes: An Updated Multilevel Meta-analysis of Randomized Controlled Trials. Diabetes Ther 15, 521–532 (2024). https://doi.org/10.1007/s13300-023-01523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01523-0