Abstract
Introduction
Recent trials have shown that glucagon-like peptide-1 receptor agonists considerably reduce atherosclerotic cardiovascular disease in patients with type 2 diabetes mellitus (T2DM). Oxidative stress, a surrogate marker of cardiovascular risk, is associated with glucose variability. However, to the best of our knowledge, no studies have compared the effects of injectable semaglutide and dulaglutide therapies on oxidative stress and glucose variability assessed via continuous glucose monitoring (CGM). This study aimed to analyze and compare the effects of semaglutide and dulaglutide therapies on oxidative stress and glucose variability as assessed through CGM.
Methods
This is an open-label, multicenter, randomized, prospective, parallel-group comparison study. Overall, 37 patients with T2DM treated with dulaglutide for at least 12 weeks were randomized into two groups: one receiving continuous dulaglutide therapy (n = 19) and one receiving injectable semaglutide therapy (n = 18) groups. The coprimary endpoints were changes in the results of the diacron-reactive oxygen metabolites test, an oxidative stress marker, and CGM-evaluated glucose variability after 24 weeks. The secondary endpoint was changes in the Diabetes Treatment Satisfaction Questionnaire (DTSQ) scores.
Results
Switching to semaglutide therapy was better than continuous dulaglutide therapy in reducing oxidative stress, glucose variability, and glycated hemoglobin levels. Conversely, continuous dulaglutide therapy was better than semaglutide therapy in terms of DTSQ scores for “Convenience” and “Recommend.”
Conclusion
Injectable semaglutide therapy may be more effective than dulaglutide therapy in ameliorating oxidative stress and regulating glucose metabolism, including glucose variability, in patients with T2DM, while dulaglutide therapy may be more effective in terms of treatment satisfaction.
Clinical Trial Registration
UMIN-CRT ID: UMIN000042670 (registered 7 December 2020).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Glucagon-like peptide-1 receptor agonist therapy (GLP-1RA) has been recently reported to considerably ameliorate atherosclerotic cardiovascular disease. |
Oxidative stress, a surrogate marker of cardiovascular risk, is linked to glucose variability. |
This is the first study to compare the effects of semaglutide therapy with another GLP-1RA (dulaglutide) on oxidative stress and glucose variability. |
What was learned from the study? |
Injectable semaglutide therapy is better than dulaglutide therapy for reducing oxidative stress and improving glucose variability. |
Patients reported higher treatment satisfaction with dulaglutide therapy than with semaglutide therapy. |
Introduction
Type 2 diabetes mellitus (T2DM) is a major risk factor for atherosclerosis. Atherosclerotic cardiovascular disease (CVD) is a leading cause of death in patients with T2DM [1, 2]. The United Kingdom Prospective Diabetes Study (UKPDS) 80 reported that intensive treatment of hyperglycemia considerably reduced the risk of myocardial infarction and death in patients with early diagnosed T2DM followed up during a 10-year post-trial period [3]. However, the Action to Control Cardiovascular Risk in Diabetes Study and Veterans Affairs Diabetes Trial revealed the limited beneficial effects of relatively short-term strict blood glucose control on cardiovascular events in patients with long-lasting T2DM with CVD history or multiple cardiovascular risk factors [4, 5]. Therefore, how and when hyperglycemia has been treated may be two important factors in preventing atherosclerotic CVD in patients with T2DM.
Recently, the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) 6 and Researching Cardiovascular Events with a Weekly Incretin in Diabetes trials have shown that therapy with glucagon-like peptide-1 receptor agonists (GLP-1RAs) decreases the risk of cardiovascular events in high-risk patients with T2DM [6, 7]. Based on the positive results of these clinical trials, the American Diabetes Association and European Association for the Study of Diabetes Consensus Report recommend the use of GLP-1RAs for treating patients with T2DM with atherosclerotic CVD and/or multiple coronary risk factors [8].
However, accumulating evidence shows that glucose variability is associated with endothelial dysfunction in patients with T2DM, an initial step for progression to atherosclerosis through oxidative stress generation, which reportedly plays a pivotal role in the development and progression of atherosclerotic CVD [9, 10]. Indeed, we have previously demonstrated that diacron-reactive oxygen metabolites (d-ROMs), a surrogate marker of oxidative stress, are correlated with glucose variability, as evaluated through continuous glucose monitoring (CGM) in patients with T2DM [11]. Furthermore, endothelial dysfunction and the presence and severity of coronary artery disease are positively associated with glucose variability in patients with T2DM. These observations suggest that increased oxidative stress and glucose variability may be therapeutic targets for atherosclerotic CVD in patients with T2DM [12, 13].
In the SUSTAIN 7 trial, injectable semaglutide therapy was more effective than dulaglutide therapy in reducing glycated hemoglobin (HbA1c) levels and body weight (BW) in patients with T2DM [14]. However, to the best of our knowledge, no clinical study has compared the effects of injectable semaglutide and dulaglutide therapies on oxidative stress and glucose variability in patients with T2DM. Additionally, we are unsure whether once-weekly dulaglutide therapy can further improve patient satisfaction in T2DM compared with once-daily liraglutide therapy [15, 16]. Therefore, the aim of this study was to compare the effects of injectable semaglutide and dulaglutide therapies on oxidative stress evaluated via d-ROMs, glucose variability assessed through CGM, and treatment satisfaction according to the Diabetes Treatment Satisfaction Questionnaire (DTSQ) scores in patients with T2DM.
Methods
Patients
Outpatients with T2DM (age ≥ 20 years) who had been treated with dulaglutide for at least 12 weeks at Showa University Hospital and Tokatsu Hospital in Japan and whose HbA1c levels were ≥ 6.5% (47 mmol/mol) were enrolled in the present study. The following patients were excluded: (1) those with an estimated glomerular filtration rate (eGFR) of ≤ 30 mL/min/1.73 m2 according to the Cockcroft–Gault formula; (2) those who were undergoing steroid and/or anti-inflammatory drug treatment, including vitamin C supplementation; (3) those with severe infection and trauma and/or perioperative period; (4) those with diabetic ketosis or those who were in coma within the 3 preceding months of entering into the study; (5) pregnant women; (6) those with malignancy; and (7) those who were deemed inappropriate for inclusion as assessed by their respective physician.
Study Design
This was an open-label, multicenter, randomized, prospective study conducted at Showa University Hospital, a private university hospital responsible for tertiary care, and Tokatsu Hospital, a general hospital providing secondary care, in Japan between April 2021 and May 2022. Figure 1 summarizes the study protocol. We randomly assigned patients to two groups using an Excel (Microsoft Corp., Redmond, WA, USA)-based allocation system with stratification and 1:1 allocation via permuted-block randomization; one group received a continuous dose of 0.75 mg/week dulaglutide therapy and the other group switched to injectable semaglutide therapy (0.25 mg/week) after dulaglutide therapy. After 4 weeks, the semaglutide dose was increased to 0.5 mg/week. At baseline and 24 weeks following the intervention, we measured the following clinical and laboratory parameters before breakfast on day 1 of CGM [17]: BW, low-density lipoprotein cholesterol (LDL-C) levels, high-density lipoprotein cholesterol (HDL-C) levels, triglyceride (TG) levels, eGFR, blood pressure, fasting plasma glucose (FPG) levels, and HbA1c levels. All clinical data, such as diabetes duration and smoking habit, were retrieved from the medical records of the patients. The patients were instructed to maintain their lifestyle and continue receiving the same dose of any concomitant drugs. Additionally, they were instructed to follow a weight-maintaining diet (25–30 kcal/kg ideal BW; 50–60% carbohydrates, 20% proteins, and the rest fat) during the study period.
All procedures were followed according to the ethical standards of the responsible committee on human experimentation (institutional and national) and/or the Helsinki Declaration of 1964 and its later versions. Informed consent or an acceptable alternative was obtained from all patients included in the study. The study was approved by the Ethics Committee of Showa University (Approval number 3292; date of approval, 19 November 2020), and was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR ID: UMIN000042670). The treatment costs were covered by insurance.
Procedures and Measurements
A FreeStyle Libre Pro CGM device (Abbott Japan, Tokyo, Japan) was subcutaneously inserted into each patient on day 1 of the study and subsequently removed from the patient on day 14. We calculated glucose variability from day 3 to day 12 to exclude inaccurate data. The coefficient of variation (%CV) was measured by dividing the standard deviation (SD) of the glucose level by the mean glucose level (MGL) and multiplying it by 100 [18]. To assess daily glucose variability, we calculated the mean amplitude of glucose excursion (MAGE) [19]. The mean of the absolute difference between the corresponding glucose values indicated the mean daily difference in blood glucose levels [20]. We also calculated the time above range (TAR), defined as the percentage of time spent at > 180 mg/dL (> 10.0 mmol/L), the time in range (TIR), defined as the percentage of time spent within the target range of 70–180 mg/dL (3.9–10.0 mmol/L) during a 24-h period, and the time below range, defined as the percentage of time spent at < 70 mg/dL (< 3.9 mmol/L) [21].
Laboratory Measurements
Oxidative stress was measured using the d-ROMs test, which evaluates free radical activity by measuring the serum levels of hydroperoxides according to previous reports [22, 23]. The results of the d-ROMs test are expressed in arbitrary units (i.e., as Caratelli Units [U.CARR], where 1 U.CARR = the oxidant capacity of 0.08 mg/dL H2O2 solution and the normal range was 250–300 U.CARR). Moreover, clinical variables were measured using an automated analyzer (model BM6070; Japan Electron Optics Laboratory, Tokyo, Japan). The plasma glucose levels were measured using the glucose oxidase method, and HbA1c levels were determined by high-performance liquid chromatography [24].
Diabetes Treatment Satisfaction and Eating Behavior
To assess treatment satisfaction, we used the DTSQ, which is a validated self-administered questionnaire, and calculated its scores at baseline and 24 weeks after the intervention [25, 26]. The DTSQ comprises eight items scored on a 7-point Likert scale ranging from 0 (very dissatisfied) to 6 (very satisfied). The scores of six satisfaction items, namely “Current treatment,” “Convenience,” “Flexibility,” “Understanding,” “Recommend,” and “Continue,” were summed up, with 36 as the highest possible total score. The two remaining items, “Perceived frequency of hyperglycemia” and “Perceived frequency of hypoglycemia,” were evaluated individually.
For assessing eating behavior, we used the questionnaire of the Guideline for Obesity issued by the Japan Society for the Study of Obesity at baseline and 24 weeks after the start of the study. This questionnaire consists of 55 items with seven major scales [27, 28].
Endpoints and Assessments
The primary endpoints were changes in d-ROMs and glucose variability from baseline after completing the 24-week treatment intervention. The secondary endpoint was changes in the DTSQ score.
Sample Size Calculation
As mentioned above, to our knowledge, no clinical studies have yet compared the effects of dulaglutide and injectable semaglutide therapies on glucose variability and oxidative stress. Therefore, we determined the sample size after reviewing the studies that have investigated the effects of incretin modulators, including GLP-1RAs, on glucose variability and oxidative stress [16, 29]. Furthermore, considering the possibility of a 20% dropout rate, we calculated the sample size as 20 individuals per group.
Statistical Analysis
All normally distributed continuous data, as determined via the Shapiro–Wilk test, are expressed as the mean ± SD. Differences in continuous variables between the semaglutide and dulaglutide therapy groups at baseline and after treatment were evaluated using independent samples t-test or Mann–Whitney U-test, as appropriate. To compare categorical variables, we used the Chi-square test. A p value of < 0.05 was to be considered statistically significant. All the statistical data were analyzed using SPSS version 22 for Windows (IBM Corp., Armonk, NY, USA).
Results
Patient Sample and Characteristics
Figure 2 shows the flowchart of patient inclusion. We screened 42 patients, of whom one declined to participate and two did not meet the inclusion criteria. Of the 39 remaining patients, 19 were randomized to the semaglutide therapy group and 20 to the dulaglutide therapy group. However, one patient from each group was removed because they experienced pain from injection and were lost to follow-up, ultimately leaving 18 and 19 patients in the semaglutide and dulaglutide therapy groups, respectively, for analysis (Fig. 2). Table 1 lists the baseline clinical characteristics. The mean age, diabetes duration, and HbA1c levels of the semaglutide and dulaglutide therapy groups were 72.2 ± 9.7 and 71.8 ± 11.3 years, 18.2 ± 11.1 and 20.3 ± 10.7 years, and 7.7% ± 0.7% and 7.4% ± 0.6%, respectively. The clinical characteristics and biochemical parameters at baseline were not significantly different between the two groups.
Effects of Semaglutide and Dulaglutide Therapies on Clinical and Biochemical Parameters
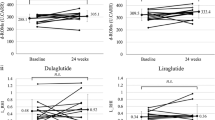
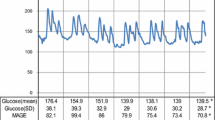
Table 2 summarizes the clinical and biochemical parameters. After 24 weeks of treatment, changes in clinical and biochemical parameters from baseline were not significantly different between the two groups, except for oxidative stress and glucose metabolism. Compared to the dulaglutide group, the semaglutide therapy group exhibited better changes in d-ROMs (mean ± SD, 4.4 ± 60.5 vs. − 36.1 ± 57.6 U.CARR, respectively; p = 0.045) and HbA1c levels (0.2% ± 0.6% vs. − 0.4% ± 0.5%, respectively; p = 0.006). Figure 3 shows the 24-h blood glucose profiles as assessed by CGM. The mean (± SD) changes in SD, MAGE, %CV, TAR, and TIR were also better in the semaglutide therapy group than in the dulaglutide therapy group (SD: − 4.3 ± 10.1 vs. 5.3 ± 9.3 mg/dL, p = 0.005; MAGE: − 6.7 ± 21.1 vs. 12.2 ± 22.8 mg/dL, p = 0.013; %CV: − 2.8% ± 4.6% vs. 0.1% ± 3.3%, p = 0.038; TAR: − 4.0 ± 22.5 vs. 11.3 ± 20.8 mg/dL, p = 0.039; TIR;4.7 ± 22.2 vs. − 10.4 ± 18.6 mg/dL, p = 0.031). Clinical and biochemical parameters, including MGL, MAGE, and TAR, were unaffected by the switch to semaglutide therapy, whereas they worsened significantly by continuous dulaglutide therapy for 24 weeks (from 140.2 ± 20.9 to 158.0 ± 28.6 mg/dL, p = 0.035; from 95.4 ± 15.7 to 107.6 ± 20.1 mg/dL, p = 0.045; from 18.6% ± 13.8% to 30.3% ± 19.4%, p = 0.045, respectively).
Table 3 shows the correlations between the changes in d-ROMs and glucose metabolism parameters. In the dulaglutide therapy group, no statistically significant relationship was observed between changes in d-ROMs and glucose metabolism parameters. In the semaglutide therapy group, changes in d-ROMs tended to be associated with changes in SD (r = 0.420, p = 0.083) and TIR (r = − 0.401, p = 0.099). When the data of both the groups were combined, changes in d-ROMs were significantly correlated with HbA1c (r = 0.338, p = 0.040), MGL (r = 0.349, p = 0.034), SD (r = 0.430, p = 0.008), MAGE (r = 0.333, p = 0.044), CV (r = 0.325, p = 0.050), TAR (r = 0.373, p = 0.023), and TIR (r = − 0.404, p = 0.013) values in the univariate analysis. In the multivariate stepwise regression analysis, only SD appeared to be correlated with d-ROMs (β = 0.430, p = 0.008) (adjusted multiple R2 = 0.162).
Effects of Semaglutide and Dulaglutide on DTSQ Scores
Table 4 summarizes the self-reported patient treatment satisfaction as evaluated via the DTSQ scores and eating behavior at baseline and 24 weeks following the treatment. Although DTSQ scores did not significantly improve with either of the treatments, changes in “Convenience” and “Recommend” from baseline significantly differed between the dulaglutide and semaglutide therapy groups (“Convenience”: − 0.5 ± 1.3 vs. 0.7 ± 1.4, p = 0.005; “Recommend”: − 0.2 ± 1.1 vs. 1.1 ± 2.1, p = 0.045). After 24 weeks of treatment, there were no changes in either group regarding the eating behavior scores.
Discussion
To the best of our knowledge, this clinical study is the first to compare the effects of injectable semaglutide and dulaglutide therapies on oxidative stress and glucose metabolism as measured via CGM, patient satisfaction, and eating behavior. The results of this study demonstrated that switching to injectable semaglutide therapy was more effective than continuous dulaglutide therapy in reducing an oxidative stress marker, d-ROMs, and glucose variability in patients with T2DM, whereas continuous dulaglutide therapy demonstrated higher treatment satisfaction than its counterpart.
In studies on patients with T2DM who were on diet and exercise therapy, dulaglutide and liraglutide therapies reportedly improved the daily glucose variability [30, 31]. However, we have previously reported that once-weekly dulaglutide therapy is better than once-daily liraglutide therapy in terms of controlling the daily glucose variability [16]. In the present study, we compared the effects of therapy with another once-weekly GLP-1 RA, i.e., semaglutide, with those of dulaglutide therapy and showed that semaglutide therapy was more effective than dulaglutide therapy in reducing the daily glucose variability following a 24-week treatment. Although there was no statistically significant difference in the FPG levels of patients treated with 0.5 mg/week semaglutide and 0.75 mg/week dulaglutide, semaglutide therapy demonstrated more favorable effects on HbA1c levels. Furthermore, while changes in HbA1c levels were not correlated with FPG (r = 0.258, p = 0.301), they were correlated with the other glucose metabolism parameters, such as MGL (r = 0.537, p = 0.021), SD (r = 0.500, p = 0.034), MAGE (r = 0.533, p = 0.023), TAR (r = 0.595, p = 0.009), and TIR (r = − 0.599, p = 0.009). In the SUSTAIN 7 trial, 0.5 mg/week semaglutide therapy considerably reduced the mean blood glucose levels, postprandial hyperglycemia across all meals, and mean 7-point self-measured blood glucose levels compared with the 0.75 mg/week dulaglutide therapy [14]. Therefore, semaglutide therapy may improve glucose variability with greater efficiency than dulaglutide therapy by reducing postprandial hyperglycemia in patients with T2DM.
Overgaard et al. reported that 0.5 mg/week semaglutide therapy resulted in higher circulating concentrations of GLP-1 than 0.75 mg/week dulaglutide therapy [32]. GLP-1RAs may have direct beneficial effects on oxidative stress because the cAMP–PKA pathway evoked by GLP-1RAs inhibited the NAD(P)H oxidase–derived oxidative stress generation [33, 34]. Furthermore, in experimental animal models, higher circulating concentrations of GLP-1 demonstrated more favorable effects on atherosclerotic lesion progression by suppressing oxidative stress [35, 36]. Owing to its favorable pharmacokinetic profile, switching to semaglutide therapy may reduce oxidative stress more efficiently than continuous dulaglutide therapy. Moreover, it is conceivable that GLP-1RAs decreased oxidative stress by improving glucose metabolism parameters, including glucose variability. Indeed, improvements in glucose variability were reportedly correlated with reduction in oxidative stress in patients with T2DM [37]. In the present study, a decrease in the oxidative stress marker d-ROMs was correlated with improvement in SD, one of the markers of daily glucose variability. Therefore, switching to semaglutide therapy may decrease oxidative stress more effectively by improving glucose variability compared to continuous dulaglutide therapy.
GLP-1 slows down gastric emptying and suppresses appetite by promoting satiety and inhibiting hunger through the central nervous system, thereby leading to BW loss [38, 39]. In the SUSTAIN 7 trial, injectable semaglutide therapy reduced BW more in comparison with dulaglutide therapy [14]. However, in the present study we found that BW loss did not show significant differences between the semaglutide and dulaglutide therapy groups. The discrepant results on BW between the SUSTAIN 7 trial and the present study could be ascribed partially to the differences in the study design; the former compared the effects of semaglutide and dulaglutide therapies in patients with GLP-1 RA–naïve T2DM, whereas we compared the effects of switching to semaglutide therapy and continuous dulaglutide therapy. Lucas et al. reported that injectable semaglutide therapy resulted in greater BW loss in patients with GLP-1 RA–naïve T2DM than in those who had already been treated with other GLP-1RAs and then switched to injectable semaglutide therapy [40]. In addition, switching from dulaglutide therapy to injectable semaglutide therapy has a weaker BW-reducing effect than switching from other GLP-1RAs [41].
In terms of the DTSQ scores for “Convenience” and “Recommend,” continuous dulaglutide therapy was better compared to switching to injectable semaglutide therapy. Additionally, the total scores of DTSQ, which reflect treatment satisfaction, tended to be higher in the dulaglutide therapy group than in the semaglutide therapy group. We used 29G needles for drug delivery in the semaglutide and dulaglutide therapy groups. A single-dose pen-injector (SPI) for semaglutide therapy is approved and used for patients with T2DM in Japan, whereas a multidose pen-injector (MPI) is used in other countries [42]. The mean visual analog scale score for pain intensity at the injection site has been reported to be higher with the SPI than with the MPI [42]. Furthermore, Matza et al. reported that patients with T2DM preferred an injection device for dulaglutide therapy to an MPI device for semaglutide therapy; more participants perceived that the device for dulaglutide therapy was easier to use than the device for semaglutide therapy [43]. These points may explain why DTSQ scores for “Convenience” and “Recommend” were higher in patients with T2DM who continued dulaglutide therapy than in those who received semaglutide therapy in place of dulaglutide therapy.
This study has several limitations. First, the sample size was small and not based on a statistical power calculation. The sample size was determined by reviewing previous studies that had investigated the effects of incretin modulators, including GLP-1RAs, on glucose variability and oxidative stress [16, 29]. Therefore, this limitation may result in potential changes in clinical and biochemical parameters being undetected. Second, the study period was relatively short (24 weeks); hence, we did not know the long-term effects of injectable semaglutide and dulaglutide therapies on oxidative stress and glucose variability in patients with T2DM. Third, the process of switching from dulaglutide therapy to another GLP-1RA may have induced a positive psychological change that could cause higher DTSQ scores in the semaglutide group. Therefore, to address these potential problems, a longitudinal observation study is required to clarify whether there are clinical differences in oxidative stress and glucose variability between patients with T2DM receiving injectable semaglutide and dulaglutide therapies. Fourth, although we did not justify the intervention dosage in this study, the standard dosage of injectable semaglutide therapy in Japan is 0.5 mg/week, and a dulaglutide therapy dosage of 0.75 mg/week is the only approved dosage for treating patients with T2DM in Japan.
Conclusion
We found that compared with dulaglutide therapy, injectable semaglutide therapy could exhibit better efficacy in ameliorating oxidative stress and glucose metabolism, including daily glucose variability, in patients with T2DM, while dulaglutide therapy may be better in improving treatment satisfaction as evaluated via DTSQ scoring.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–18.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30.
American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S125–43.
Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67.
Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–54.
Ohara M, Fukui T, Ouchi M, et al. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2016;122:62–70.
Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1–7.
Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:1–9.
Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–86.
Takase T, Nakamura A, Yamamoto C, et al. Improvement in treatment satisfaction after switching from liraglutide to dulaglutide in patients with type 2 diabetes: a randomized controlled trial. J Diabetes Investig. 2019;10:699–705.
Nagaike H, Ohara M, Kohata Y, et al. Effect of dulaglutide versus liraglutide on glucose variability, oxidative stress, and endothelial function in type 2 diabetes: a prospective study. Diabetes Ther. 2019;10:215–28.
Ohara M, Nagaike H, Fujikawa T, et al. Effects of omarigliptin on glucose variability and oxidative stress in type 2 diabetes patients: a prospective study. Diabetes Res Clin Pract. 2021;179:108999.
Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832–8.
Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–55.
Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–8.
American Diabetes Association Professional Practice Committee. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S83-96.
Gerardi G, Usberti M, Martini G, et al. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002;40:104–10.
Cesarone MR, Belcaro G, Carratelli M, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–30.
Schnedl WJ, Lahousen T, Wallner SJ, Krause R, Lipp RW. Silent hemoglobin variants and determination of HbA(1c) with the high-resolution program of the HPLC HA-8160 hemoglobin analyzer. Clin Biochem. 2005;38:88–91.
Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7:445–51.
Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. In: Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. London: Harwood Academic Publishers; 1994. p. 111–32.
Inoue K, Maeda N, Kashine S, et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:109.
Lau KK, Wong YK, Chan YH, et al. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:101.
Sagara M, Suzuki K, Aoki C, et al. Impact of teneligliptin on oxidative stress and endothelial function in type 2 diabetes patients with chronic kidney disease: a case-control study. Cardiovasc Diabetol. 2016;15:76.
Inoue M, Shiramoto M, Oura T, Nasu R, Nakano M, Takeuchi M. Effect of once-weekly dulaglutide on glucose levels in Japanese patients with type 2 diabetes: findings from a phase 4, randomized controlled trial. Diabetes Ther. 2019;10:1019–27.
Mori Y, Taniguchi Y, Sezaki K, Yokoyama J, Utsunomiya K. Liraglutide narrows the range of circadian glycemic variations in Japanese type 2 diabetes patients and nearly flattens these variations in drug-naive type 2 diabetes patients: a continuous glucose monitoring-based study. Diabetes Technol Ther. 2011;13:1139–44.
Overgaard RV, Lindberg SØ, Thielke D. Impact on HbA1c and body weight of switching from other GLP-1 receptor agonists to semaglutide: a model-based approach. Diabetes Obes Metab. 2019;21:43–51.
Hendarto H, Inoguchi T, Maeda Y, et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012;61:1422–34.
Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–50.
Kushima H, Mori Y, Koshibu M, et al. The role of endothelial nitric oxide in the anti-restenotic effects of liraglutide in a mouse model of restenosis. Cardiovasc Diabetol. 2017;16:122.
Koshibu M, Mori Y, Saito T, et al. Antiatherogenic effects of liraglutide in hyperglycemic apolipoprotein E-null mice via AMP-activated protein kinase-independent mechanisms. Am J Physiol Endocrinol Metab. 2019;316:E895-907.
Ohara M, Nagaike H, Goto S, et al. Improvements of ambient hyperglycemia and glycemic variability are associated with reduction in oxidative stress for patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;139:253–61.
van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–96.
Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt WE, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–32.
Yale JF, Bodholdt U, Catarig AM, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care. 2022;10:e002619.
Crabtree TSJ, Adamson K, Reid H, et al. Injectable semaglutide and reductions in HbA1c and weight in the real world in people switched from alternative glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2022;24:1398–401.
Snitker S, Andersen A, Lindskov PS, van Marle S, Sode BF, Sparre T. Comparison of the injection-site experience of semaglutide in a single-dose and a multidose pen-injector. Diabetes Obes Metab. 2022;24:1643–6.
Matza LS, Boye KS, Stewart KD, et al. Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: a crossover study (PREFER). Diabetes Obes Metab. 2020;22:355–64.
Acknowledgements
We thank the participants in this study. We also thank Ms. Tomomi Saito at Showa University; Ms. Yasue Moroto, Ms. Rie Tomaru, and Ms. Nanami Koizumi at the Showa University Hospital; and Ms. Shiho Amano, Ms. Mari Fujinami, Ms. Sachie Terakado, Ms. Tomoko Yamada, Ms. Yukari Otani, and Ms. Ayu Koketsu at Tokatsu Hospital for their respective assistance. Further, we would like to thank Enago (http://www.enago.jp) for providing English language review of this article.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Study design, data acquisition, and data analysis and manuscript drafting: Takemasa Omachi, Makoto Ohara. Review and editing of the manuscript for intellectual content: Yusaku Mori, Tomoyasu Fukui, and Sho-Ichi Yamagishi. Manuscript drafting: Takemasa Omachi, Makoto Ohara, and Sho-Ichi Yamagishi. Data interpretation and critically revision and drafting of the manuscript: Takemasa Omachi, Makoto Ohara, Tomoki Fujikawa, Yo Kohata, Hiroe Sugita, Shunichiro Irie, Michishige Terasaki, Yusaku Mori, Tomoyasu Fukui, and Sho-Ichi Yamagishi. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Makoto Ohara has received lecture fees from Eli Lilly Japan K.K. Yusaku Mori holds an endowed chair funded by Ono Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. Sho-Ichi Yamagishi has received lecture fees from Eli Lilly Japan K.K, Bayer Yakuhin, Ltd., Sanofi K.K., and Novo Nordisk Pharma, Japan. Sho-Ichi Yamagishi, Tomoyasu Fukui, Yo Kohata, Hiroe Sugita, Takemasa Omachi, Shunichiro Irie, Michishige Terasaki and Tomoki Fujikawa declare that they have no conflicts of interest.
Ethical Approval
All procedures were followed according to the ethical standards of the responsible committee on human experimentation (institutional and national) and/or the Helsinki Declaration of 1964 and later versions. Informed consent or an acceptable alternative was obtained from all patients included in the study. The study was approved by the Ethics Committee of Showa University (approval number 3292; date of approval, 19 November 2020), and was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000042670). The treatment costs were covered by insurance.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Omachi, T., Ohara, M., Fujikawa, T. et al. Comparison of Effects of Injectable Semaglutide and Dulaglutide on Oxidative Stress and Glucose Variability in Patients with Type 2 Diabetes Mellitus: A Prospective Preliminary Study. Diabetes Ther 15, 111–126 (2024). https://doi.org/10.1007/s13300-023-01493-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01493-3