Abstract
Introduction
This study aimed to develop a simplified screening model to identify pregnant Chinese women at risk of gestational diabetes mellitus (GDM) in the first trimester.
Methods
This prospective study included 1289 pregnant women in their first trimester (6–12 weeks of gestation) with clinical parameters and laboratory data. Logistic regression was performed to extract coefficients and select predictors. The performance of the prediction model was assessed in terms of discrimination and calibration. Internal validation was performed through bootstrapping (1000 random samples).
Results
The prevalence of GDM in our study cohort was 21.1%. Maternal age, prepregnancy body mass index (BMI), a family history of diabetes, fasting blood glucose levels, the alanine transaminase to aspartate aminotransferase ratio (ALT/AST), and the triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C) were selected for inclusion in the prediction model. The Hosmer–Lemeshow goodness-of-fit test showed good consistency between prediction and actual observation, and bootstrapping indicated good internal performance. The area under the receiver operating characteristic curve (ROC-AUC) of the multivariate logistic regression model and the simplified clinical screening model was 0.825 (95% confidence interval [CI] 0.797–0.853, P < 0.001) and 0.784 (95% CI 0.750–0.818, P < 0.001), respectively. The performance of our prediction model was superior to that of three other published models.
Conclusion
We developed a simplified clinical screening model for predicting the risk of GDM in pregnant Chinese women. The model provides a feasible and convenient protocol to identify women at high risk of GDM in early pregnancy. Further validations are needed to evaluate the performance of the model in other populations.
Trial Registration
ClinicalTrials.gov identifier: NCT03246295.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Gestational diabetes mellitus (GDM) is a common complication of pregnancy, and the prevalence of GDM has increased in the past few years. However, a feasible method to screen GDM risk in early pregnancy is lacking. |
We aimed to develop a convenient clinical prediction model to identify pregnant women at high risk of GDM in early pregnancy that could be applicable in most areas of China. |
What was learned from the study? |
Three clinical characteristics (maternal age, prepregnancy body mass index, and a family history of diabetes) and three laboratory parameters (fasting blood glucose level, the triglyceride to high-density lipoprotein cholesterol ratio, and the alanine transaminase to aspartate aminotransferase ratio) in the first trimester were selected and used to develop a simplified clinical screening model. The model showed good discrimination (ROC-AUC 0.784, 95% confidence interval 0.750–0.818, P < 0.001) and calibration. |
The simplified prediction model in our study provided a simple and feasible tool to predict the risk of GDM in early pregnancy. The performance of our prediction model was superior to that of three other published models, and our prediction model would be applicable in pregnant Chinese women. |
Introduction
Gestational diabetes mellitus (GDM) is a common complication of pregnancy and is defined as a condition of glucose intolerance that is first diagnosed during pregnancy [1]. The prevalence of GDM has increased globally in the past few years, possibly because of the rapid societal transitions in nutrition and lifestyles. GDM affects up to 15% of pregnant women worldwide, whereas it affects 18.3–25% of pregnant women in Southeast Asia, demonstrating the higher prevalence of GDM in China [2,3,4]. Accumulating evidence indicates that GDM can not only increase the risk of perinatal complications (pregnancy-induced hypertension, preeclampsia, stillbirth, etc.) but also lead to chronic health problems for offspring later in life, including diabetes mellitus, metabolic syndrome, and cardiovascular diseases [5, 6].
According to the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria, the diagnosis of GDM is based on the results of a 2-h, 75-g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation [7]. However, pregnant women with GDM could have hyperglycemia for a longer period of time, even during the first trimester of pregnancy; thus, the diagnosis of GDM at 24–28 weeks of gestation might be retrospective and may not completely reverse the adverse effects on both mothers and their offspring [2]. Therefore, it is essential to predict the risk of GDM in early pregnancy to improve the hyperglycemic environment.
Several risk factors, including advanced maternal age, prepregnancy body mass index (preBMI), a family history of diabetes mellitus, and glucose and lipid profiles in early pregnancy, have been applied for the early identification of GDM [8,9,10]. Based on our previous work, the triglyceride (TG) to high-density lipoprotein cholesterol (HDL-C) ratio (TG/HDL-C), alanine transaminase (ALT) to aspartate aminotransferase (AST) ratio (ALT/AST), and hepatic steatosis index (HSI) are independent risk factors for GDM [11, 12]. In recent years, other novel biomarkers have been reported as potential predictors, including angiopoietin-like protein 8 and plasma fatty acid-binding protein 4 [13, 14]. The use of individual biochemical markers has shown relatively poor sensitivity and specificity and, thus, combinations of risk factors have been taken into consideration for predicting the risk of GDM. Several studies explored the utility of preBMI combined with fasting blood glucose (FBG) in the first trimester as risk factors to predict the risk of GDM [15,16,17]. However, there were no unified cutoff values among different studies, which limited the practicability of these combined risk factors. Because of the similar pathogenesis between GDM and type 2 diabetes mellitus (T2DM), several genetic variants related to insulin secretion (including glucokinase [GCK] and melatonin receptor 1B [MTNR1B]) and insulin resistance (including insulin receptor substrate 1 [IRS1] and peroxisome proliferator-activated receptor gamma [PPARG]) have been found to be associated with GDM [18]. Although the role of genetic variants in the prediction of GDM risk has been discussed, the conclusions are inconsistent [19, 20]. To achieve early identification of the risk of GDM, there has been a rapid development of prediction models based on sociodemographic characteristics and laboratory data. However, these predictors are mostly evaluated during the second trimester (after 12 weeks of gestation), and it is uncertain whether the models developed by other regions are applicable to Chinese women [21, 22]. In addition, some of the prediction models are too complex, and the variables included in the models are not routinely tested during pregnancy [23].
The aim of the present study was to develop a convenient clinical prediction model to identify pregnant women at high risk of GDM in early pregnancy. A mathematical formula was first established by logistic regression analysis, and then a simplified screening model was derived. The diagnostic utility of our prediction model was compared with that of other published GDM prediction models.
Methods
Ethical approval
Written informed consent was obtained from each participant, and the study was performed in accordance with the Declaration of Helsinki as revised in 2013. This study was part of an ongoing prospective double-center observational cohort study initiated in 2019, which was conducted at Haidian District Maternal and Child Health Care Hospital and Chaoyang District Maternal and Child Health Care Hospital (Beijing, China) (ClinicalTrials.gov: NCT03246295). The Ethical Review Committee of National Center for Women and Children’s Health, Chinese Center for Disease Control and Prevention in Beijing, China approved this study on 3 April 2019 (approval number: FY2019-01).
Participants
Singleton pregnant women aged > 18 years were recruited to the study at their first prenatal visit during the first trimester of pregnancy (between 6 and 12 weeks). The inclusion criteria were: (1) < 12 weeks gestation, and the ability to follow-up regularly; (2) natural conception; (3) no medication use before or during pregnancy, except for vitamins; and (4) agreement to participate in the study and to provide a signed consent form. The exclusion criteria were: (1) twin or multiple pregnancy; (2) impaired glucose tolerance or diabetes mellitus before pregnancy; (3) severe chronic diseases or infectious diseases (e.g., liver disease, kidney failure, cardiovascular disease, autoimmune disease, hematological disease, AIDS, and other diseases before pregnancy); and (4) the inability to understand and complete the study. The enrollment flow chart is shown in Electronic Supplementary Material (ESM) Fig. 1. Because a previous study revealed that an FBG level ≥ 6.1 mmol/L in early pregnancy could predict the risk of GDM with a specificity of 100%, participants with an FBG level ≥ 6.1 mmol/L at the first visit were excluded from our study [10]. Baseline anthropometric and sociodemographic characteristics of the eligible women were collected at the first visit.
Clinical and Laboratory Measurements
Body height and weight were measured, and the BMI was calculated as (weight [kg])/(height [m])2. Body weight, systolic blood pressure and diastolic blood pressure were measured at each follow-up visit. Blood pressure was measured twice at 5-min intervals using an automatic BP monitor and averaged.
Laboratory tests were performed at the first visit. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (FBG [mmol/L] × fasting insulin [µU/mL])]/22.5 [24]. All participants were offered a 2-h, 75-g OGTT between 24 and 28 weeks of gestation for GDM screening. GDM was diagnosed according to the 2010 IADPSG criteria [25]. Overall, 1289 pregnant women were included in the present study. All available data were recorded and verified by two investigators simultaneously.
Data were collected on the following pregnancy outcomes from electronic medical records: gestational age at birth, type of delivery, infant birth weight, and the 10-min Apgar score. Preterm delivery was defined as delivery before gestational week 37 [26]. Large for gestational age (LGA) and small for gestational age (SGA) were defined as birth weights above the 90th percentile and below the 10th percentile of the mean weight for gestational age and sex, respectively [27]. Delivery data were available for 1064 of the 1289 participants.
Statistical Analysis
Missing data accounted for < 10% of all data, and were handled by multiple imputations of 5. Continuous variables are presented as the mean ± standard deviation if normally distributed and as medians (interquartile range) if nonnormally distributed; categorical variables are presented as percentages. Categorical variables were evaluated using the Pearson Chi-squared test (χ2). Comparisons between outcome groups for continuous variables were assessed by two-sample Student’s t-test or the Mann–Whitney U-test as appropriate.
Univariate and multivariate logistic regression analyses were performed to identify the risk factors for GDM by computing diagnostic odds ratios (ORs) and their 95% confidence intervals (95% CIs). A backward stepwise entry procedure was used to preliminarily select the variables to be retained in the multivariate logistic regression model with a statistical significance cutoff of P = 0.05. The variables included in the predictive model were selected on the basis of the Akaike information criterion. The coefficient estimates in the prediction model were normalized to construct a simplified GDM screening model. The diagnostic accuracy of the GDM prediction model and simplified screening model were evaluated by receiver operating characteristic (ROC) analysis. The optimal cutoff values were defined by obtaining the maximum Youden index calculated by the following formula: (sensitivity + specificity) − 1 [28]. The area under the curve (AUC) with the 95% CI, sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR−) were used as measures of overall performance. Calibration was evaluated by the Hosmer–Lemeshow goodness-of-fit test and internally validated with bootstrapping (1000 random samples) to reduce overfitting bias. Statistical analyses were performed using the IBM SPSS statistical program (version 26.0; SPSS IBM Corp, Armonk, NY, USA),, GraphPad Prism (version 9.5.1, GraphPad Software, San Diego, CA, USA), and R software (version 4.3.1, packages Hmisc, rms, and caret; R Foundation for Statistical Computing, Vienna, Austria). A P value of < 0.05 (two-tailed) was considered to be statistically significant.
The similar methodologies described in this study have been presented in our previous work [29].
Results
Clinical and Laboratory Characteristics
Of the 1289 participants enrolled in the present study, 272 (21.1%) developed GDM. The maternal and pregnancy characteristics of all participants are shown in Table 1. Compared to those in the normal glucose tolerance (NGT) group, women in the GDM group were older and heavier (P < 0.05). A family history of diabetes and a history of adverse pregnancy did not significantly differ between the two subgroups. The majority of participants in this study were nulliparous (64.0% and 72.4% for the GDM and NGT groups, respectively), but more women with GDM were multiparous (P = 0.024). Women who developed GDM had significantly higher levels of FBG and HOMA-IR in the first trimester of pregnancy (P < 0.01); in addition, other metabolic measures, including ALT, the ALT/AST ratio, and lipid profiles (TC, TG, HDL-C, low-density lipoprotein-cholesterol [LDL-C] levels, and the TG/HDL-C ratio) were also significantly different between the two groups (P < 0.05). Regarding pregnancy outcomes, most of the participants had a term delivery, and there was no significant difference in the incidence of preterm delivery between the GDM and NGT groups. However, the proportion of LGA was higher in the GDM group than in the NGT group (5.4% vs. 2.0%, respectively; P = 0.006).
Predictors of GDM
The potential predictors of GDM were included in the logistic regression analysis. All clinical variables were included, and laboratory variables in early pregnancy were screened to simplify the prediction model (the FBG was substituted for the HOMA-IR, the ALT/AST ratio was substituted for the ALT and AST levels, respectively, and the TG/HDL-C ratio was substituted for other lipid measures). After using the backward (LR) method for preliminary predictor selection, five variables remained in the model, including two clinical variables and three laboratory variables. Although a family history of diabetes was not significantly different between the GDM and NGT subgroups in our cohort, it has been reported to be an important risk factor for GDM in previous studies [9]. Therefore, we added a family history of diabetes to the prediction model. The univariate and multivariate logistic regression analyses for the final six variables are presented in Table 2, including the coefficients (β), ORs (95% CIs), and P values. Except for family history of diabetes, maternal age (adjusted OR 1.070, 95% CI 1.027–1.114), preBMI (adjusted OR 1.607, 95% CI 1.484–1.739), FBG (adjusted OR 1.881, 95% CI 1.425–2.482), the ALT/AST ratio (adjusted OR 3.345, 95% CI 1.969–5.683), and the TG/HDL-C ratio (adjusted OR 1.754, 95% CI 1.204–2.553) remained independent factors associated with GDM.
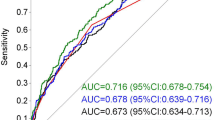
Based on the above variables included in the multivariate regression analysis, a prediction model was established. The probability (P) of GDM could be calculated according to the following formula: Logit P = − 18.263 + (0.067 × maternal age [years]) + (0.474 × preBMI [kg/m2]) − (0.088 × family history of diabetes [1 if yes, 0 if no]) + (0.632 × FBG [mmol/L]) + (1.208 × ALT/AST) + (0.562 × TG/HDL-C). ROC analysis in this prediction model showed an area under the curve (AUC) of 0.825 (95% CI 0.797–0.853, P < 0.001), with a sensitivity of 76% and a specificity of 72% (Fig. 1). This prediction model was assessed by the Hosmer–Lemeshow goodness-of-fit test and was internally validated by bootstrapping. Hosmer–Lemeshow goodness-of-fit testing indicated good consistency between the predicted and actual data (χ2 = 9.756, P = 0.283) (Fig. 2a). The calibration curve after bootstrapping indicated good internal performance in terms of discrimination, with an adjusted C-statistic of 0.821 (Fig. 2b).
Assessment of the multivariate logistic model. a Hosmer–Lemeshow goodness-of-fit test (χ2 = 9.756, P = 0.283). b Bootstrap-validated calibration curve (β = 1000 repetitions, boot, mean absolute error = 0.018, n = 1289). The x-axis represents the predicted probability of the multivariate logistic model, and the y-axis represents the actual probability of gestational diabetes mellitus. Perfect prediction would correspond to the 45° dashed line. The red line represents the entire cohort, and the orange line indicates bias correction by bootstrapping
Simplified Clinical Screening Model for GDM
In according to the CHARMS recommendations [30], we extracted coefficients from the multivariate logistic regression and used these to calculate the GDM risk score. The fitted model and simplified scores are reported in Table 3, and details on the variables included in the screening model are as follows:
-
Maternal age. The cutoff values were set according to the standards for advanced maternal age and extremely advanced maternal age [31]: age < 35 years (score of 0), age between 35 and 40 years (score of 2), and age ≥ 40 years (score of 4).
-
preBMI. The cutoff values were set according to China’s standards for overweight or obesity [32]: preBMI < 24 kg/m2 (score of 0), preBMI between 24 and 28 kg/m2 (score of 10), and preBMI ≥ 28 kg/m2 (score of 20).
-
Family history of diabetes. A family history of diabetes was defined as at least one family member having been diagnosed with diabetes, with a score of 1 for yes and a score of 0 for none.
-
Fasting blood glucose. The cutoff values were set according to the threshold for elevated blood glucose levels in the first trimester as recommended by the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) studies [33]: FBG < 5.1 mmol/L (a score of 0), FBG between 5.1 and 5.6 mmol/L (a score of 2), and FBG ≥ 5.6 mmol/L (a score of 4).
-
ALT/AST ratio. The cutoff value was set according to our previous work [12]: ALT/AST ratio < 0.825 (a score of 0) and ALT/AST ratio ≥ 0.825 (score of 5).
-
TG/HDL-C ratio. The cutoff value was set according to our previous work [12]: TG/HDL-C ratio < 0.676 (score of 0) and TG/HDL-C ratio ≥ 0.676 ( score of 3).
ROC curves were used to analyze the performance and discrimination of the simplified screening model (Fig. 1). The simplified screening model had an AUC of 0.784 (95% CI 0.750–0.818, P < 0.001), demonstrating a well-accepted predictive and discriminative performance. The optimal cutoff of the scoring model was 5.5, with a sensitivity of 71% and a specificity of 74%; the LR+) was 2.73, and the LR− was 0.39. As shown in ESM Table 1, when the cutoff point was ≥ 12.5, the specificity of GDM prediction was > 95%; when the cutoff point was ≥ 18.5, the specificity of GDM prediction was > 99%. The diagnostic capacity of this prediction model at different cutoff points is described in Fig. 3 and ESM Table 1.
Sensitivity Analysis by Different preBMI Cutoff Values
As the findings of previous studies suggested lower preBMI cutoff values for application in pregnant Chinese women [16, 17], we used different preBMI cutoff values ranging from 21 to 24 kg/m2 for overweight stratification (Fig. 4; ESM Table 3). When the preBMI cutoff value was 22 kg/m2, the ROC-AUC of our prediction model was 0.789 (0.756–0.821); the two other preBMI cutoff values (21 and 23 kg/m2) did not show better ROC-AUC values than the cutoff value of 24 kg/m2. The pairwise comparisons of different preBMI cutoff values did not show statistically significant differences (P > 0.05).
Comparison of the Performance of Our model with other GDM Prediction Models
The performance of our model was compared with that of other prediction models published in the last 10 years. The screening and selection process of these models are given in ESM Fig. 2. Of the 886 records retrieved through the database search, we selected three published clinical risk models to compare with our model [34,35,36]. As shown in Fig. 1 and ESM Table 2, our current model was superior to the other established GDM prediction models, with AUCs of 0.752 (95% CI 0.721–0.784) for Gao et al.’s model [34], 0.672 (95% CI 0.636–0.708) for Zheng et al.’s model [35], and 0.736 (95% CI 0.704–0.768) for Guo et al.’s model [36]. For two of the three published models (those of Gao et al. and Guo et al.), the performance in our participants was better than the original models, whereas in Zheng et al.’s model it was worse.
Discussion
In the present study, we developed a simplified clinical screening model for predicting the risk of GDM in early pregnancy. Using three clinical characteristics (maternal age, preBMI, and a family history of diabetes) and three laboratory parameters (FBG, the ALT/AST ratio, and the TG/HDL-C ratio) measured in the first trimester, the model showed good discrimination (a sensitivity of 71% and a specificity of 74%, with an AUC of 0.784) and calibration (as shown in Fig. 2a, b). This prediction model provided earlier screening for the risk of GDM, which would be applicable in pregnant Chinese women.
Pregnant women with GDM have an increased risk of pregnancy complications. A systematic review and meta-analysis including 156 studies revealed that women with GDM had increased odds of cesarean section, preterm delivery, macrosomia, and LGA infants. Among pregnant women with GDM requiring insulin therapy, the odds of having an infant with respiratory distress syndrome were also higher [37]. Based on the results of these studies, it is necessary to identify the risk of GDM as early as possible. Although numerous risk factors for GDM have been reported, the ability to precisely identify women at high risk for GDM before or early in pregnancy remains limited. The IADPSG recommended using an FBG range of 5.1–6.9 mmol/L before 24 weeks of gestation to define early GDM, and pregnant women with FBG levels in this range should be referred for immediate intervention [25]. However, it has been reported that FBG is related to gestational age and body weight, and several women with GDM have normal FBG levels in early pregnancy [38]. In addition, one study reported that even among pregnant women with FBG levels > 5.6 mmol/L before 24 weeks of gestation, > 50% did not develop GDM, indicating that it was inaccurate to predict the risk of GDM by FBG levels alone [39]. Heterogeneity of physiological processes underlying hyperglycemia has been revealed among women with GDM [40]. In a proportion of pregnant women with GDM, the pathophysiological mechanism of GDM was dominated by insulin secretion defects without impaired insulin sensitivity, whereas other patients had predominant insulin sensitivity defects with hyperinsulinemia and were more likely to develop altered adipokine profiles. The association between lipid profiles and liver function in early pregnancy and GDM has gradually been elucidated, but the diagnostic ability of each study was different with disparate cutoff points [41, 42]. Our previous work identified clinically useful biomarkers in early pregnancy for the prediction of GDM risk, which were used as variables in the prediction model reported in the present study and to determine cutoff values [11, 12]. The parameters included in our scoring model have been reported in previous studies, providing the theoretical basis of the model.
Race is one of the risk factors for GDM [9]. The incidence of GDM in Chinese individuals is significantly higher than that in white individuals; thus, prediction models based on European or North American populations are not applicable for Chinese women. Several GDM prediction models have been established in China. Wu et al. developed a clinical model for gestational women in the first trimester by selecting seven variables via advanced machine learning, which demonstrated a promising predictive value [43]. However, the model was too complicated to use in routine clinical care, especially in rural areas. Wang et al. found that FBG and TG levels during gestational weeks 14–20 were independent predictors for GDM and built a risk score using these two variables [44]. The prediction model based on laboratory data ignored the relationship between sociodemographic characteristics and GDM. More studies devoted to predicting the risk of GDM by novel biomarkers, including genetic variants and proteomic analysis, have been implemented in most institutions in China [45, 46]. The aim of this study was to establish a practical and propagable method to identify the risk of GDM in Chinese women in early pregnancy, and the simplified screening model presented herein achieved high accuracy. Three published models with variables similar to ours were contrasted with our prediction model, but none of them had better predictive values than our model, neither the original AUC values nor the derived ones [34,35,36].
The diagnostic utility of our prediction model was satisfactory, with an AUC of 0.784 (95% CI 0.750–0.818, P < 0.001). The optimal cutoff value of the model was 5.5, with a sensitivity of 71% and a specificity of 74%, which indicates that it could be a simplified and low-cost screening tool for clinical use. As shown in ESM Table 1, when the cutoff point was ≥ 12.5, the specificity was > than 95%; when the cutoff point was ≥ 18.5, the specificity was > than 99%. Therefore, we recommend that if the score is > 12.5, intervention measures should be taken immediately because of the high probability of GDM. In addition, women with FBG levels ≥ 6.1 mmol/L in the first trimester were excluded from our prediction model. Patients with FBG levels ≥ 6.1 mmol/L were defined as having impaired fasting glucose (IFG), which indicated that they may already have abnormal glucose metabolism. Zhu et al. found that an fasting plasma glucose cutoff values of 6.1 mmol/L at the first prenatal visit had a specificity of 1 for predicting the risk of GDM [10]. Based on the above, we recommend that pregnant women with an FBG level ≥ 6.1 mmol/L in the first trimester should be treated as women with GDM and receive lifestyle intervention or even insulin treatment.
There are several limitations to our study. First, some missing data were missing during early pregnancy in this prospective cohort. However, the proportion of missing data was < 10%, and multiple imputations were conducted to develop the prediction model. Second, as our study was derived and internally validated only in pregnant Chinese women, it may not be applicable to other populations. Performing external validation in other populations and different settings would have been the optimal approach, but this was not feasible in this cohort. Moreover, although the screening model showed good discrimination, it could not identify all women at high risk of GDM in the first trimester. When the cutoff point was 5.5, the screening model failed to identify 78 of the 272 (28.6%) pregnant women with GDM in this study. Further studies on GDM risk factors are needed to establish more accurate prediction models.
Conclusions
In conclusion, we developed a simplified screening model that can predict the risk of GDM in early pregnancy in the Chinese population based on sociodemographic characteristics and laboratory data; this model is easy to implement in most medical centers in China. The diagnostic utility of our prediction model showed better discrimination than other published models using similar biomarkers, with an ROC-AUC of 0.784 (95% CI 0.750–0.818). This model could help identify women at high GDM risk earlier than the 75-g OGTT, which may reduce the rate of perinatal complications in pregnant women as well as the economic burden of society.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Gabbe SG. Gestational diabetes mellitus. N Engl J Med. 1986;315(16):1025–6.
Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909.
Wang C, Jin L, Tong M, et al. Prevalence of gestational diabetes mellitus and its determinants among pregnant women in Beijing. J Matern Neonatal Med. 2020;21:1–7.
Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–85.
Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diab Rep. 2019;19:143.
Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–9.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S13–27.
Wang C, Zhu W, Wei Y, et al. The predictive effects of early pregnancy lipid profiles and fasting glucose on the risk of gestational diabetes mellitus stratified by body mass index. J Diabetes Res. 2016;2016:3013567.
Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59(7):1403–11.
Zhu WW, Yang HX, Wei YM, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care. 2013;36(3):586–90.
Song S, Duo Y, Zhang Y, et al. The predictive ability of hepatic steatosis index for gestational diabetes mellitus and large for gestational age infant compared with other noninvasive indices among Chinese pregnancies: a preliminary double-center cohort study. Diabetes Metab Syndr Obes. 2021;16(14):4791–800.
Song S, Zhang Y, Qiao X, et al. ALT/AST as an independent risk factor of gestational diabetes mellitus compared with TG/HDL-C. Int J Gen Med. 2022;5(15):115–21.
Leong I. Diabetes: ANGPTL8 as an early predictor of gestational diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):64.
Ning H, Tao H, Weng Z, Zhao X. Plasma fatty acid-binding protein 4 (FABP4) as a novel biomarker to predict gestational diabetes mellitus. Acta Diabetol. 2016;53(6):891–8.
Popova PV, Grineva EN, Gerasimov AS, Kravchuk EN, Ryazantseva EM, Shelepova ES. The new combination of risk factors determining a high risk of gestational diabetes mellitus. Minerva Endocrinol. 2015;40(4):239–47.
Li P, Yin Y, Lin S, et al. Utility of pregestational body mass index and initial fasting plasma glucose in predicting gestational diabetes mellitus. Am J Med Sci. 2016;351(4):420–5.
Hao M, Lin L. Fasting plasma glucose and body mass index during the first trimester of pregnancy as predictors of gestational diabetes mellitus in a Chinese population. Endocr J. 2017;64(5):561–9.
Zhang C, Bao W, Rong Y, et al. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum Reprod Update. 2013;19(4):376–90.
Popova PV, Klyushina AA, Vasilyeva LB, et al. Association of common genetic risk variants with gestational diabetes mellitus and their role in GDM prediction. Front Endocrinol (Lausanne). 2021;19(12): 628582.
Rosta K, Al-Aissa Z, Hadarits O, et al. Association study with 77 SNPs confirms the robust role for the rs10830963/G of MTNR1B variant and identifies two novel associations in gestational diabetes mellitus development. PLoS ONE. 2017;12(1): e0169781.
White SL, Lawlor DA, Briley AL, et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLoS ONE. 2016;11(12): e0167846.
Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018;145:130–7.
Sweeting AN, Wong J, Appelblom H, et al. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther. 2019;45(2):76–84.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82.
Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–25.
Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS ONE. 2014;9(8): e104779.
Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med. 2014;33(6):985e1000.
Duo Y, Song S, Zhang Y, et al. Predictability of HOMA-IR for gestational diabetes mellitus in early pregnancy based on different first trimester BMI values. J Pers Med. 2022;13(1):60.
Moons KG, de Groot JA, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
Kahveci B, Melekoglu R, Evruke IC, Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. 2018;18(1):343.
Zhou BF, on behalf of the Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002.
Gao S, Leng J, Liu H, et al. Development and validation of an early pregnancy risk score for the prediction of gestational diabetes mellitus in Chinese pregnant women. BMJ Open Diabetes Res Care. 2020;8(1): e000909.
Zheng T, Ye W, Wang X, et al. A simple model to predict risk of gestational diabetes mellitus from 8 to 20 weeks of gestation in Chinese women. BMC Pregnancy Childbirth. 2019;19(1):252.
Guo F, Yang S, Zhang Y, Yang X, Zhang C, Fan J. Nomogram for prediction of gestational diabetes mellitus in urban, Chinese, pregnant women. BMC Pregnancy Childbirth. 2020;20(1):43.
Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;25(377):e067946.
Cosson E, Carbillon L, Valensi P. High fasting plasma glucose during early pregnancy: a review about early gestational diabetes mellitus. J Diabetes Res. 2017;2017:8921712.
Popova P, Tkachuk A, Dronova A, et al. Fasting glycemia at the first prenatal visit and pregnancy outcomes in Russian women. Minerva Endocrinol. 2016;41(4):477–85.
Powe CE, Allard C, Battista MC, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052–5.
An R, Ma S, Zhang N, et al. AST-to-ALT ratio in the first trimester and the risk of gestational diabetes mellitus. Front Endocrinol (Lausanne). 2022;29(13):1017448.
Wang Y, Huang Y, Wu P, et al. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: a prospective nested case-control study in Chinese women. Am J Clin Nutr. 2021;114(5):1763–73.
Wu YT, Zhang CJ, Mol BW, et al. Early prediction of gestational diabetes mellitus in the Chinese population via advanced machine learning. J Clin Endocrinol Metab. 2021;106(3):e1191–205.
Wang Y, Ge Z, Chen L, et al. Risk prediction model of gestational diabetes mellitus in a Chinese population based on a risk scoring system. Diabetes Ther. 2021;12(6):1721–34.
Wu Q, Chen Y, Zhou M, et al. An early prediction model for gestational diabetes mellitus based on genetic variants and clinical characteristics in China. Diabetol Metab Syndr. 2022;14(1):15.
Wang X, Zhao M, Guo Z, et al. Urinary proteomic analysis during pregnancy and its potential application in early prediction of gestational diabetes mellitus and spontaneous abortion. Ann Transl Med. 2022;10(13):736.
Medical Writing and Editorial Assistance
The English editorial assistance of the manuscript was provided by Hannah S of Springer Nature Author Services, and we express our gratitude for their help. The English editorial assistance was funded by the first author Yanbei Duo.
Funding
This study was supported by 13th Five-Year National Science and Technology Major Project for New Drugs (Grant No. 2019ZX09734001 to WZ). The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Yanbei Duo, Tao Yuan, Weigang Zhao, Wei Sun, and Ailing Wang conceptualized the study. Yuemei Zhang, Shuoning Song, Jiyu Xu, Yan Chen, Xiaorui Nie, Qiujin Sun, Xianchun Yang, and Zechun Lu performed the investigation. Yanbei Duo, Xiaolin Qiao, Zhenyao Peng, Jing Zhang, Tao Yuan, Yong Fu, and Yingyue Dong determined the methodology. Yanbei Duo, Shuoning Song, Yuemei Zhang, Xiaolin Qiao, Jiyu Xu, and Yan Chen collected the clinical data. Yanbei Duo wrote the original draft. Yanbei Duo, Tao Yuan, Weigang Zhao, Wei Sun, and Ailing Wang edited the manuscript. Weigang Zhao supervised the study. All authors approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Yanbei Duo, Shuoning Song, Xiaolin Qiao, Yuemei Zhang, Jiyu Xu, Jing Zhang, Zhenyao Peng, Yan Chen, Xiaorui Nie, Qiujin Sun, Xianchun Yang, Ailing Wang, Wei Sun, Yong Fu, Yingyue Dong, Zechun Lu, Tao Yuan, and Weigang Zhao have nothing to disclose.
Ethical Approval
Written informed consent was obtained from each participant, and the study was performed in accordance with the Declaration of Helsinki as revised in 2013. This study was part of an ongoing prospective double-center observational cohort study started in 2019, which was conducted at Haidian District Maternal and Child Health Care Hospital and Chaoyang District Maternal and Child Health Care Hospital (Beijing, China) (ClinicalTrials.gov: NCT03246295). The Ethical Review Committee of National Center for Women and Children’s Health, Chinese Center for Disease Control and Prevention in Beijing, China approved this study on 3 April 2019 (approval number: FY2019-01).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Duo, Y., Song, S., Qiao, X. et al. A Simplified Screening Model to Predict the Risk of Gestational Diabetes Mellitus in Pregnant Chinese Women. Diabetes Ther 14, 2143–2157 (2023). https://doi.org/10.1007/s13300-023-01480-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01480-8