Abstract
Early and intensive treatment of type 2 diabetes (T2D) has been associated with lower risk of diabetes-related complications. Control of overweight and obesity, which are strongly associated with T2D and many of its complications, is also key in the management of the disease. New therapies allow for individualised glycaemic control targets with greater safety. Thus, in patients with a higher cardiovascular and renal risk profile, current guidelines encourage early treatment with metformin together with glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose co-transporter-2 inhibitors with proven cardiovascular benefit. GLP-1 RAs combine highly efficacious glucose-lowering activity with a reduced risk of hypoglycaemia. Recently, tirzepatide, a first-in-class drug that activates both glucose-dependent insulinotropic polypeptide and GLP-1 receptors, has demonstrated very high efficacy in glycated haemoglobin (HbA1c) and weight reduction in clinical trials. Tirzepatide has the potential to help people with T2D reach recommended glycaemic and weight targets (HbA1c < 7% and > 5% weight reduction) and to allow some patients to reach HbA1c measurements close to normal physiological levels and substantial weight reduction. In 2022, tirzepatide was approved by the US Food and Drug Administration and the European Medicines Agency for treatment of people with T2D and is currently in development for chronic weight management.
Plain Language Summary
In people newly diagnosed with type 2 diabetes, early and intensive treatment of the disease can help control blood sugar and reduce the risk of later complications. The need to control weight in people with obesity and diabetes has also recently become a priority. New drugs developed in recent years allow for better and more individualised management of blood sugar without the risk of blood sugar levels dropping too low. In patients at risk of kidney or heart disease, the current recommendation is early treatment with metformin and drugs with proven cardiovascular benefit. Tirzepatide is a new drug that has also demonstrated very high efficacy in reducing blood glucose and body weight. It has the potential to help people with type 2 diabetes achieve their goals and prevent other diabetes-related complications. It is likely that some patients will even be able to bring their blood glucose to normal levels and lose substantial amounts of weight. The US and European regulatory agencies approved tirzepatide in 2022 for the treatment of type 2 diabetes and it is currently being tested for chronic weight management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In people newly diagnosed with type 2 diabetes (T2D), early and intense intervention to improve glycaemic control can prevent diabetes-related micro- and macrovascular complications in the long term |
In people with T2D and excess weight or obesity, weight loss can help achieve glycaemic control by reversing the underlying metabolic causes of the disease, but sustained weight reduction is often difficult to achieve |
Glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose co-transporter-2 inhibitors with proven cardiovascular and renal benefits are currently recommended for T2D treatment, in combination with other therapies for people at cardiovascular and renal risk |
Tirzepatide is a new drug that activates both glucose-dependent insulinotropic polypeptide and GLP-1 receptors and, in clinical trials, has demonstrated very high efficacy in both glycaemic and weight control. It is now approved for T2D treatment and is in development for chronic weight management |
As new treatments are incorporated into the diabetes treatment, cost-effectiveness studies are increasingly necessary to better target interventions and to inform decision making on reimbursement and pricing |
Tirzepatide has the potential to help people with T2D reach the recommended glycaemic and weight targets and to help some patients achieve normoglycaemia and substantial weight reduction |
Introduction
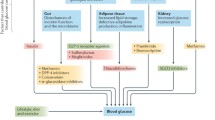
Type 2 diabetes (T2D) remains, for most people, a life-long, chronic disease with associated complications. The current focus of T2D management is on early intervention, with the objective of avoiding acute metabolic decompensation and preventing or delaying the onset of the cardiovascular or renal complications characteristically associated with diabetes. The general recommendations for people newly diagnosed with T2D are in favour of healthy lifestyle changes, including better nutrition and more physical exercise. Thus, the American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” currently recommends a glycated haemoglobin (HbA1c) goal for nonpregnant adults of < 7% (53 mmol/mol), but lower HbA1c levels may be acceptable and even beneficial if they can be achieved safely without significant hypoglycaemia or other adverse effects of treatment [1]. In all cases, it is critical that the individualisation of targets is based on key patient characteristics, such as the patient’s risk factors and comorbidities. Pharmacological therapies, such as some glucagon-like peptide-1 receptor agonists (GLP-1 RAs) or sodium-glucose transport protein-2 (SGLT2) inhibitors with proven cardiovascular benefit, which combine glucose control with a low risk of hypoglycaemia, are currently accepted as appropriate initial therapy with or without metformin based on glycaemic needs for individuals with T2D with or at high risk for atherosclerotic cardiovascular disease (CVD), heart failure and/or chronic kidney disease [2, 3].
As obesity has been strongly associated with the development and progression of T2D and many of its associated complications, in recent years weight reduction has been proposed as a primary target of management for people with overweight or obesity with T2D [3,4,5,6]. To achieve weight reduction goals, the recent consensus by the ADA-European Association for the Study of Diabetes (EASD) recommends individualised weight loss goals and consideration of GLP-1 RAs with high weight loss efficacy, as they can often provide weight loss of 10–15% or more [3]. New therapies, such as tirzepatide, have demonstrated very high efficacy for weight reduction [7].
In this review, we discuss the impact of stringent glucose control and weight reduction on health outcomes when achieved early in the disease course along with the current and future pharmacological therapies aimed at achieving these goals.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Benefits of Tight and Early Glycaemic Control
Several studies have shown that early intervention in the form of behavioural or lifestyle changes, pharmacological therapy or surgery can be successful in reverting diabetes and achieving stringent glycaemic control in some patients [8,9,10,11]. For example, long-lasting remissions in up to 50% of patients have been observed in studies of patients newly diagnosed with T2D, HbA1c > 8.5–9% and clinical symptoms who underwent early initiation of insulin [12]. In these cases, the key determinant of the likelihood of inducing sustained drug-free diabetes remission was early intervention, particularly within the first 2 years after diagnosis [13]. Baseline body mass index (BMI) and fasting plasma glucose were clinical predictors of success in these patients [12].
Glycaemic control has proven effective in avoiding the toxic effects of hyperglycaemia and the development of micro- and macrovascular complications [3]. Some long-term studies (UKPDS, UKPDS 80, ADVANCE, ORIGIN, Diabetes and Aging Study) have shown the significant benefit of early insulin treatment (Table 1) [14,15,16,17,18,19,20]. In some cases, a reduction in cardiovascular events and microvascular disease was observed, and improved life expectancy was observed in two studies with follow-up of > 10 years. The ORIGIN study demonstrated that initiating intensive insulin therapy at diagnosis of T2D to achieve stringent glycaemic control reversed glucotoxicity, resulting in recovery of residual β-cell function, but did not show benefit on the prevention of micro- or macrovascular complications or mortality between the groups [12]. More recently, a cohort study of managed care patients newly diagnosed with T2D and 10 years of survival data found that diabetes control during the first year after diagnosis was strongly associated with lower future risk for diabetic complications and mortality. Interestingly, the duration and intensity of early glycaemic control were both closely aligned with outcomes [20].
In conclusion, some studies with long follow-up times, such as UKPDS, have demonstrated the beneficial effects of early intervention to improve glycaemic control and long-term complications.
Benefits of Weight Control
Obesity is a chronic relapsing progressive disease process with a critical role in the development and progression of T2D and many of its associated complications [4, 5, 21]. For these reasons, there is renewed interest in weight control and the concept of weight-centric, rather than glucose-centric, approaches to diabetes treatment [21]. Obesity is strongly associated with insulin resistance, hyperinsulinaemia and glucose intolerance and increased rates of cardiovascular events, microvascular complications and other diabetes-derived comorbidities [22]. Men with obesity had a seven-fold higher risk and women with obesity a 12-fold higher risk of developing T2D compared with individuals in the healthy weight range [22]. Weight gain has been associated with strong increases in the risk of development of prediabetes or diabetes and in the reduction of the rate of reversion to normoglycaemia in subjects with prediabetes [23, 24].
The DiRECT study, a randomised, controlled trial, evaluated an intensive dietary intervention in patients with recent T2D (< 6 years of duration) and a BMI of 27–45 kg/m2 [25]. In this study, 70% of patients who lost ≥15 kg achieved T2D remission by 2 years [24]. Similarly, 60% of those who lost between 10 and 15 kg, 29% of those who lost between 5 and 10 kg and 5% of those who lost < 5 kg at 2 years had diabetes remission, suggesting a direct relationship between weight loss and diabetes improvement [24]. Weight reduction can also help improve CVD risk factors in individuals with T2D. The Look AHEAD trial of 5145 patients with overweight and obesity showed that a modest weight decrease of 5% to < 10% was associated with significant reductions in blood pressure and triglycerides and higher high-density lipoprotein cholesterol after 1 year [26]. Weight losses of a higher magnitude (> 10%) were significantly associated with a lower risk of cardiovascular events, such as cardiovascular death, myocardial infarction, stroke or angina hospitalisation [27].
Significant weight loss (10–15%) can be disease modifying and lead to full T2D remission in some patients [3]. Very-low-calorie diets can result in rapid weight loss and major improvements to glycaemic control and remission, but currently they are usually reserved for individuals with higher obesity degree (BMI > 35 kg/m2) [28]. In addition, although weight loss can help improve glucose control in T2D by reversing the underlying metabolic causes of the disease, achieving healthy weight goals can be very difficult for a high percentage of patients [24, 26]. Additional research is needed to evaluate these lifestyle interventions.
Early Intensification with Combination Therapy Versus Sequential Treatment
Until recently, stepwise drug treatment intensification has been the standard approach, mostly because of the increased risk of hypoglycaemia, and provides a clear evaluation of the efficacy of new drugs and their potential side effects [29]. Recent evidence shows that starting with a combination of metformin and a GLP-1 RA or SGLT2 inhibitor in newly diagnosed patients can lead to earlier, better and more sustained glucose control [30, 31]. Older studies also support this view. The EDICT randomised trial tested treatment with a combination of metformin, pioglitazone and a GLP-1 RA (exenatide) in patients newly diagnosed with T2D versus sequential add-on therapy. This study found that significantly more participants initially receiving combination therapy maintained the treatment goal (HbA1c < 6.5%) and had HbA1c reduced to the normal range (< 6.0%) than those receiving conventional sequential therapy [32]. Early treatment intensification was also supported by randomised studies of patients treated with metformin and a dipeptidyl peptidase-4 inhibitor [33, 34], metformin and a sulphonylurea [35] and other drug groups [36].
Current ADA-EASD consensus recommendations indicate that combination treatment can be considered in some patients with newly diagnosed T2D to extend the time to treatment failure, especially in patients presenting with HbA1c levels 1.5–2.0% above target [3]. In young adults with T2D, immediate and sustained glycaemic control (HbA1c levels of ≤7% or even lower) should be the goal [3]. In summary, intensification with combination therapy of high glucose-lowering efficacy or therapies for cardiovascular/renal risk reduction such as GLP-1 RAs and SGLT2 inhibitors could be advantageous over sequential addition to better individualise treatments [2].
GLP-1 RAs and SGLT2 Inhibitors in Patients with Cardiovascular Risk
In the past decade, seven cardiovascular outcome trials have provided consistent data on the efficacy of some GLP-1 RAs and SGLT2 inhibitors in reducing cardiovascular events in people with T2D. Meta-analyses of the cardiovascular outcomes trials show that some GLP-1 RAs can reduce major cardiovascular events, cardiovascular death, myocardial infarction rates and stroke, among other benefits [37, 38]. Likewise, strong evidence of reduction in major cardiovascular events and hospital admissions for heart failure and cardiovascular death has been observed for some SGLT2 inhibitors [39, 40]. For these reasons, the ADA, the EASD and the European Society of Cardiology published recommendations for the prescription of GLP-1 RAs and SGLT2 inhibitors with proven cardiovascular or renal benefits to patients with T2D and established CVD or high cardiovascular risk [2, 3, 41]. Some current guidelines also suggest that GLP-1 RAs and SGLT2 inhibitors with proven cardiovascular or renal benefits should be administered as first-line monotherapy in patients with atherosclerotic CVD or with high cardiovascular risk and that the decision to treat with these drugs should be considered independently of baseline HbA1c or individualised HbA1c target [2, 41]. Early initiation with combination therapy based on metformin and a GLP-1 RA or SGLT2 inhibitor could be an option for most people newly diagnosed with T2D [31]. Also, a significant advantage of GLP-1 RA and SGLT2 inhibitor treatments is their effect on body weight. Although the extent of the loss varies, there is substantial evidence from clinical trials on the weight-loss effects of some of these drugs [42]. Furthermore, recent studies have shown that some GLP-1 RAs and SGLT2 inhibitors could have the potential for treating non-alcoholic fatty liver disease and non-alcoholic steatohepatitis, two conditions with currently very limited therapeutic options [43, 44]. However, there are still no randomised clinical trials with conclusive evidence to support this indication for use.
Although GLP-1 RAs and SGLT2 inhibitors are generally well tolerated, the long-term safety of these drugs (> 10-years) has not been evaluated.
The fact that some patients with established cardiovascular disease are not being treated with these drugs, although they are recommended in clinical guidelines, is a cause of concern for some researchers and authors of guidelines [45,46,47]. Authors of the ADA-EASD consensus guidelines have suggested that clinical inertia could be a reason for this gap between clinical evidence and clinical practice [47]. Others suggest inadequate recognition by doctors that they may be used for cardiovascular benefit regardless of glycaemic control or the fact that these new agents are more expensive compared with older drugs [3, 46]. For policy makers, healthcare systems, payers and companies with marketed products, ensuring access should be a priority [3, 47]. New strategies have been proposed to make expensive drugs more affordable [48], but to offer innovative drugs to patients in an environment of limited economic resources, public health pharmaceutical strategies and specific proposals from the pharmaceutical sector may be needed as well as prioritisation of those groups of patients at highest risk [3, 47]. Although numerous studies have highlighted the need for decreasing the gap between guideline recommendations and clinical practice in patients with increased cardiovascular risk at earlier stages of T2D, the effort should involve healthcare providers, the pharmaceutical industry, regulators, professional societies and payers [45,46,47,48,49,50]. Also, cost-effectiveness studies of these drugs are needed to assess their clinical benefit in relation to costs. Cost-effectiveness studies would help to better target interventions and to inform decision making on reimbursement and pricing [3, 50].
Tirzepatide
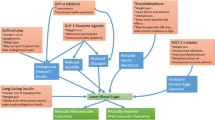
Tirzepatide is a novel once-weekly injectable single-peptide molecule with glucose-dependent insulinotropic polypeptide and GLP-1 RA activity. The combined action at both receptors may act synergistically, providing additional effects on glycaemic control and body weight reduction [51, 52].
In the SURPASS-1 to -5 clinical trials, the efficacy of tirzepatide in people with T2D was investigated as monotherapy versus placebo (in patients with mean disease duration of 4.7 years) [53]; as add-on to metformin versus semaglutide [54]; as add-on to metformin ± SGLT2 inhibitors versus insulin degludec [55, 56]; in patients with high cardiovascular risk, as add-on to metformin, SGLT2 inhibitors or sulphonylureas versus insulin glargine [57]; and as add-on to metformin plus insulin glargine versus placebo [58] (Tables 2 and 3). In SURPASS-2, tirzepatide showed robust, dose-dependent reductions in HbA1c levels (− 2.30% with the 15 mg dose after 40 weeks of treatment compared with −1.86% with semaglutide 1 mg), as well as large reductions in body weight (− 12.4 kg with the 15 mg dose, compared with − 6.2 kg with semaglutide 1 mg) [54]. Between 85 and 92% of patients treated with tirzepatide 5 mg, 10 mg or 15 mg achieved glycaemic control, defined as HbA1c < 7%, and substantial proportions of participants achieved HbA1c < 5.7% (Table 2) [53,54,55, 57, 58]. In a sub-study of SURPASS-3 in patients with continuous glucose monitoring, those receiving tirzepatide had a greater proportion of time in tight target range (71–140 mg/dl) than did those receiving insulin degludec [59].
The effect of tirzepatide on body weight was progressive and dose dependent; between 54 and 88% of patients achieved ≥ 5% weight loss across SURPASS-1 to -5 [53,54,55, 57, 58]. Also, in these trials up to 69% of patients achieved a more ambitious goal of ≥ 10% weight loss, and up to 43% experienced weight loss of ≥ 15% (Table 3). The body weight reduction was mostly due to reduced fat mass [60]. A subanalysis of patients responding to tirzepatide in the SURPASS-1 to -4 trials showed that those achieving HbA1c < 5.7% were slightly younger and had a shorter T2D duration and lower HbA1c at baseline [61]. A recent post hoc analysis of the SURPASS-1 to -5 trials showed that significantly more participants treated with tirzepatide (all doses) achieved the triple objective of an HbA1c < 5.7% with ≥ 5% weight loss and without hypoglycaemia compared with those receiving placebo, semaglutide 1 mg or basal insulin [62]. Using tirzepatide 15 mg, > 40% of patients achieved this triple endpoint. Tirzepatide (all doses) was also associated with clinically significant reductions in blood pressure and non-high-density lipoprotein cholesterol and triglyceride levels [53,54,55, 57]. Furthermore, tirzepatide (10 mg and 15 mg) was associated with a significant reduction in liver fat content and visceral and abdominal subcutaneous adipose tissue volumes compared with insulin degludec in a subpopulation of the SURPASS-3 study [63].
Tirzepatide presented a safety profile similar to that of GLP-1 RAs, mainly consisting of mild-to-moderate gastrointestinal events and no increased risk of hypoglycaemia [54]. GLP-1 RAs decelerate gastric emptying, curb postmeal glycaemic increments and reduce appetite, energy intake and body weight. A study showed that tirzepatide has similar effects [65]. However, because tirzepatide is a new drug, there are still some evidence gaps that will require additional research. For example, there are still no published data on its effect in real-world clinical conditions, long-term safety data or its use in populations beyond those studied in clinical trials. Likewise, although tirzepatide is associated with improvement of several cardiovascular risk factors (e.g., blood pressure, lipid profile, abdominal circumference), the long-term study of cardiovascular outcomes is still underway. The SURPASS-CVOT trial (NCT04255433) is currently investigating the efficacy and safety of tirzepatide compared with the GLP-1 RA dulaglutide in preventing major cardiovascular events. Finally, long-term cost-effectiveness studies are necessary to evaluate the economic impact of the introduction of tirzepatide.
In summary, tirzepatide is a new single molecule with glucose-dependent insulinotropic polypeptide and GLP-1 RA activity that has demonstrated efficacy superior to that of comparators in terms of HbA1c and weight reduction. Tirzepatide was included in the recent ADA-EASD consensus as a drug with very high efficacy in achieving glycaemic targets and high potential in weight reduction [2, 3]. In 2022, tirzepatide was approved by the US Food and Drug Administration and by the European Medicines Agency for the treatment of T2D and is currently in development for chronic weight management.
Conclusions and Prospects
In recent years, the increase in the number of therapies with specific benefits has allowed a more personalised approach to the treatment of T2D. Optimal treatment pathways should consider and evaluate the risk profile of the patient, especially their cardiovascular and renal risks. Current evidence suggests that early and intensive treatment is associated with a better chance of achieving glucose control and a lower risk of complications. Early combination treatment with metformin and other drugs, including GLP-1 RAs and SGLT2 inhibitors, can have beneficial outcomes, such as adequate glucose control and weight loss, while minimising the risk of hypoglycaemic events. The use of the drugs in these classes with proven cardiovascular benefit should be strongly encouraged in combination with metformin or as monotherapy in patients with established CVD or high cardiovascular risk. As these new drugs are more expensive than the older agents, cost-effectiveness studies are needed to improve allocation of resources and to guide reimbursement and pricing. Tirzepatide, a newly approved drug for the treatment of T2D, may allow robust glucose control and weight reduction in a single drug with weekly administration.
Data availability
This is review and no data were used. Therefore the statement is not applicable.
References
ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-110.
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140–57.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–23.
Pillon NJ, Loos RJF, Marshall SM, Zierath JR. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell. 2021;184(6):1530–44.
Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399:394–405.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16.
Dugan J, Cantillep A, Newberry K, Shubrook J. A call to action on prediabetes. JAAPA. 2018;31:26–30.
McInnes N, Hall S, Sultan F, et al. Remission of type 2 diabetes following a short-term intervention with insulin glargine, metformin, and dapagliflozin. J Clin Endocrinol Metab. 2020;105:dgaa248.
Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetologia. 2021;64:2359–66.
McInnes N, Hall S, Hramiak I, et al. Remission of type 2 diabetes following a short-term intensive intervention with insulin glargine, sitagliptin, and metformin: results of an open-label randomized parallel-design trial. Diabetes Care. 2022;45:178–85.
Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther. 2016;7:187–201.
Kramer CK, Zinman B, Choi H, Retnakaran R. Predictors of sustained drug-free diabetes remission over 48 weeks following short-term intensive insulin therapy in early type 2 diabetes. BMJ Open Diab Res Care. 2016;4:e000270.
UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Patel A, MacMahon S, ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Ritsinger V, Malmberg K, Mårtensson A, Rydén L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol. 2014;2:627–33.
Gerstein HC, Bosch J, ORIGIN Trial Investigators, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28.
ORIGIN Trial Investigators. Characteristics associated with maintenance of mean A1C<6.5% in people with dysglycemia in the ORIGIN trial. Diabetes Care. 2013;36:2915–22.
Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019;42:416–26.
Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2021;399:394–405.
Wilding JPH. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68:682–91.
Nakasone Y, Miyakoshi T, Sato Y, et al. Impact of weight gain on the evolution and regression of prediabetes: a quantitative analysis. Eur J Clin Nutr. 2017;71:206–11.
Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344–55.
Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–51.
Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6.
Gregg E, Jakicic J, Blackburn G, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–21.
Juray S, Axen KV, Trasino SE. Remission of type 2 diabetes with very low-calorie diets - a narrative review. Nutrients. 2021;13:2086.
Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137–45.
Prattichizzo F, La Sala L, Ceriello A. Two drugs are better than one to start T2DM therapy. Nat Rev Endocrinol. 2020;16:15–6.
Mosenzon O, Del Prato S, Schechter M, et al. From glucose lowering agents to disease/diabetes modifying drugs: a “SIMPLE” approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20:92.
Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015;17:268–75.
Matthews DR, Paldánius PM, Proot P, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394:1519–29.
Ji L, Chan JCN, Yu M, et al. Early combination versus initial metformin monotherapy in the management of newly diagnosed type 2 diabetes: an East Asian perspective. Diabetes Obes Metab. 2021;23:3–17.
Desai U, Kirson NY, Kim J, et al. Time to treatment intensification after monotherapy failure and its association with subsequent glycemic control among 93,515 patients with type 2 diabetes. Diabetes Care. 2018;41:2096–104.
Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:410–7.
Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85.
Marsico F, Paolillo S, Gargiulo P, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41:3346–58.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes Rev. 2019;20:816–28.
Mantovani A, Petracca G, Beatrice G, et al. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites. 2021;11:73–87.
Mantovani A, Petracca G, Csermely A, et al. Sodium-glucose cotransporter-2 inhibitors for treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Metabolites. 2020;11:22–34.
Nelson AJ, Ardissino M, Haynes K, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10:e016835.
Draznin B, Hirsch IB. Time to follow the evidence: glycemic control and cardiovascular benefits of new diabetes medications. Am J Med. 2021;134:420–2.
European Federation of Pharmaceutical Industries and Associations. Access to medicines. https://www.efpia.eu/about-medicines/access-to-medicines/. Accessed 24 June 2023.
European Commission. Making medicines more affordable. https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe/making-medicines-more-affordable_en. Accessed 24 June 2023.
Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9:46–52.
National Institute for Health and Care Excellence. Technology appraisal guidance. https://www.nice.org.uk/About/What-we-do/Our-Programmes/NICE-guidance/NICE-technology-appraisal-guidance. Accessed 24 June 2023.
Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12:143–57.
Bhagavathula AS, Vidyasagar K, Tesfaye W. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized phase II/III trials. Pharmaceuticals (Basel). 2021;14:991.
Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–55.
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15.
Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–98.
Khoo B, Tan TM-M. Surpassing insulin glargine in type 2 diabetes with tirzepatide. Lancet. 2021;398(10313):1779–81.
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;S0140–6736:02188–97.
Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327:534–45.
Battelino T, Bergenstal RM, Rodríguez A, et al. Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10:407–17.
Heise T, De Vries JH, Coskun T, et al. 338-OR: tirzepatide reduces appetite, energy intake, and fat mass in people with T2D. Diabetes. 2022;71(Suppl 1):338-OR.
Rosenstock J, Del Prato S, Franco DR, et al. Characterization of tirzepatide-treated patients achieving HbA1c <5.7% in the SURPASS 1–4 trials. In: American Diabetes Association, 82nd Annual Scientific Sessions. 2022, June 3–7; New Orleans, Louisiana, USA.
Cheng A, Lingvay I, Choudhary P, et al. Patients achieving a HbA1c < 5.7% with ≥ 5% weight loss and without hypoglycemia: a post hoc analysis of SURPASS-1 to -5. In: American Diabetes Association, 82nd Annual Scientific Sessions. 2022, June 3–7; New Orleans, Louisiana, USA.
Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10:393–406.
Mellbin LG, Rydén L, ORIGIN Trial Investigators, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013;34:3137–44.
Urva S, Coskun T, Loghin C, et al. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP-1 receptor agonists. Diabetes Obes Metab. 2020;22(10):1886–91.
Medical Writing, Editorial and Other Assistance
Francisco López de Saro and Sheridan Henness (Rx Communications, Mold, UK) provided medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and company.
Funding
Sponsorship for this review and the Rapid Service Fee was funded by Lilly SA (Spain).
Author information
Authors and Affiliations
Contributions
Luis Alberto Vasquez, Irene Romera, Miriam Rubio-de Santos and Javier Escalada met the authorship criteria and continued substantially to the conception and design, analysis and interpretation of data, and drafting of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Luis Alberto Vázquez is a former employee and minor shareholder of Eli Lilly and Company and has participated as speaker, advisor and investigator for Eli Lilly and Company, NovoNordisk, Astra-Zeneca and Boehringer-Lilly. Irene Romera and Miriam Rubio-de Santos are employees and minor shareholders of Eli Lilly and Company. Javier Escalada has participated as speaker, advisor and investigator for Astra-Zeneca, Boehringer, Eli Lilly and Company, NovoNordisk and Sanofi.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vázquez, L.A., Romera, I., Rubio-de Santos, M. et al. Glycaemic Control and Weight Reduction: A Narrative Review of New Therapies for Type 2 Diabetes. Diabetes Ther 14, 1771–1784 (2023). https://doi.org/10.1007/s13300-023-01467-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01467-5