Abstract
Introduction
Time in range (TIR) as assessed by continuous glucose monitoring (CGM) measures an individual’s glucose fluctuations within set limits in a time period and is increasingly used together with HbA1c in patients with diabetes. HbA1c indicates the average glucose concentration but provides no information on glucose fluctuation. However, before CGM becomes available for patients with type 2 diabetes (T2D) worldwide, especially in developing nations, fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) are still the common biomarkers used for monitoring diabetes conditions. We investigated the importance of FPG and PPG to glucose fluctuation in patients with T2D. We used machine learning to provide a new estimate of TIR based on the HbA1c, together with FPG and PPG.
Methods
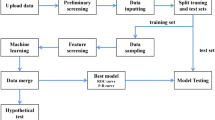
This study included 399 patients with T2D. (1) Univariate and (2) multivariate linear regression models and (3) random forest regression models were developed to predict the TIR. Subgroup analysis was performed in the newly diagnosed T2D population to explore and optimize the prediction model for patients with different disease history.
Results
Regression analysis suggests that FPG was strongly linked to minimum glucose, while PPG was strongly correlated with maximum glucose. After FPG and PPG were incorporated into the multivariate linear regression model, the prediction performance of TIR was improved compared with the univariate correlation between HbA1c and TIR, and the correlation coefficient (95% CI) increased from 0.62 (0.59, 0.65) to 0.73 (0.72, 0.75) (p < 0.001). The random forest model significantly outperformed the linear model (p < 0.001) in predicting TIR through FPG, PPG and HbA1c, with a stronger correlation coefficient 0.79 (0.79, 0.80).
Conclusions
The results offered a comprehensive understanding of glucose fluctuations through FPG and PPG compared to HbA1c alone. Our novel TIR prediction model based on random forest regression with FPG, PPG, and HbA1c provides a better prediction performance than the univariate model with solely HbA1c. The results indicate a nonlinear relationship between TIR and glycaemic parameters. Our results suggest that machine learning may have the potential to be used in developing better models for understanding patients’ disease status and providing necessary interventions for glycaemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
TIR is an important metric in CGM for interpreting individuals’ glucose fluctuations. However, it can be difficult to obtain when CGM’s accessibility is limited. A reliable method for estimating TIR is needed to facility the glycaemic management for patients with T2D. |
What was learned from the study? |
In the absence of CGM, the FPG and PPG have been shown to be effective alternative indicators for estimating TIR, in addition to HbA1c. |
The proposed TIR prediction model, which utilizes random forest regression and incorporates FPG, PPG and HbA1c as indicators, demonstrates superior prediction performance compared with previously reported models. This novel approach holds great potential as a reliable and effective tool for accurately estimating TIR levels. |
Introduction
Glycaemic variability control is closely associated with the occurrence of micro- and macrovascular diabetic complications [1]. Rapid postprandial glucose elevation and impaired glucose tolerance are risk factors for abnormal vascular function leading to serious cardiovascular diseases. Hypoglycaemia, especially nocturnal hypoglycaemia, is the most common adverse effect of diabetes therapy and is associated with unfavourable health outcomes. Poor control of hypoglycaemia will also lead to cardiovascular morbidity and mortality. It was reported that 2–4% of people with diabetes die from hypoglycaemia. The management of hypoglycaemia in individuals with diabetes has been recognized as a crucial aspect of ensuring safe and effective diabetic therapy [2]. However, patients with well-controlled HbA1c levels (< 6%) were found to have an increased risk of hypoglycaemia. HbA1c has long been the gold standard for assessing long-term glycaemic management. It has been demonstrated to have a significant correlation with the mean glucose levels measured by continuous glucose monitoring (CGM) over a certain period of time [3]. However, HbA1c falls short in reflecting glucose fluctuations, e.g. hyper- and hypoglycaemia, and individuals with the same HbA1c may exhibit significantly different diurnal glucose variations [4]. A previous study found that the risk of cardiovascular complications in patients with T2D is high even in the absence of elevated HbA1c, suggesting that fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) are risk factors for cardiovascular disease [5,6,7]. The VADT study pointed out that the risk of all-cause mortality risk was elevated even with a significant decline of HbA1c level [8]. The results of the VADT study indicated that HbA1c is not a sufficient indicator of prognosis in diabetes, particularly with regard to hypoglycaemia and postprandial hyperglycaemia, and considered the underlying causes of glucose fluctuations [9].
The fasting and 2-h postprandial glycaemic levels commonly assessed through oral glucose tolerance test (OGTT) or mixed-meal glucose tolerance test (MMTT) have been recommended as an alternative diagnostic measurement for T2D alongside HbA1c [10].
The level of FPG has been reported as a marker of nocturnal hypoglycaemia [11]. PPG, on the other hand, was recommended as an effective measurement for evaluating meal-related glucose fluctuations and diabetes treatment [12, 13]. These results indicate that the levels of FPG and PPG from traditional glycaemic measurements might be closely correlated with parameters of glycaemic variability [14].
The monitoring and alarming of hypo- or hyperglycaemic events can be facilitated through the continuous and real-time recording of glucose fluctuations by the CGM system. Time in range (TIR), as the main CGM metric, can capture the percentage of time that an individual’s glucose is between 70 and 180 mg/dl, along with time below range (TBR) and time above range (TAR), and it shows daily fluctuations in glucose and the amount of time spent high or low across several days. It was reported to be closely correlated with the incidence of hypo- and hyperglycaemia, as the rise and decline of blood glucose outside of the target range can be reflected by the TIR level [15, 16]. TIR provides a comprehensive picture of glucose control within a period of days, as it takes into account both the frequency and duration of hyper- and hypoglycaemic readouts. This information can be more useful and actionable than HbA1c in short-term glycaemic variability management, helping individuals with diabetes and clinicians personalize their management strategies [17]. Poor control of TIR has been reported to be associated with many diabetic complications in the Chinese population, including diabetic retinopathy, osteoporosis, cardiovascular disease, and others [18,19,20]. This highlights TIR as a novel perspective for improving clinical outcomes and the cost-effectiveness of diabetes healthcare economics. By incorporating TIR into diabetic care, healthcare providers can optimize patient care by addressing specific glycaemic events and improving overall glucose management.
Despite the potential benefits of using CGM for monitoring glycaemic variations, its adoption in routine clinical practice, particularly in primary care, remains limited [21]. China has the highest number of patients with diabetes globally, with approximately 141 million people suffering from the disease. It is predicted that this number will increase to 174 million by 2045 [10]. At the same time, 42% of the population reside in rural areas where the medical resources and economic conditions are limited. The circumstance is even worse in other developing and undeveloped countries [22]. This has generated a huge demand for CGM in diabetes management, but it also poses very large challenges regarding clinician awareness and cost of reimbursement. Nevertheless, widespread adoption of CGM in large-scale trials still faces challenges due to various factors [23], such as the difference in time spent wearing the device, and CGM data are often difficult to compare between studies [24,25,26]. Until now, CGM data have not been a major indicator for regulators in large-scale trials [26, 27]. However, the glucose tolerance tests are more acceptable than CGM even in undeveloped areas. In China, the Guideline for the Prevention and Treatment of Type 2 Diabetes recommends using an OGTT for the diagnosis of T2D and a mixed-meal glucose tolerance test for disease management. The fasting and postprandial glucose levels obtained from OGTT are considered the primary criteria for diagnosing diabetes, and monitoring postprandial hyperglycaemia is recommended in all stages and types of T2D management [28]. And the cost for tests is often covered by healthcare insurance.
Studies have investigated the relationship of TIR with various clinical biomarkers and attempted to infer the TIR value through alternative glucose profiles when CGM data are not available [5, 29, 30]. The main clinical method to investigate TIR derived from HbA1c is univariate regression analysis, which has been widely adopted in estimating the changes in TIR through changes in HbA1c [30, 31]. Vigersky revealed the linear relationship between HbA1c and CGM-derived or self-monitored blood glucose (SMBG)-derived TIR and suggested a conversion table between HbA1c and TIR [31]. The conversion table is currently the most commonly used method in clinical settings when TIR is not available. However, the applicability and accuracy of the conversion table have yet to be determined.
There is no study that considers the diverse glycaemic variation information represented by TIR in exploring the relationship between HbA1c and TIR or a prediction model for TIR through a combination of FPG, PPG and HbA1c values. The FPG and PPG concentrations measured during a glucose tolerance test have provided a high-quality dataset as well as a more comprehensive understanding of an individual’s glucose fluctuations. They can be informative in the estimation of TIR [5, 32].
The aim of this study was to investigate the relationship between the CGM-derived glycaemic profile and FPG and PPG concentrations measured by the glucose tolerance test and to develop a more comprehensive prediction model with novel machine learning approaches for TIR estimation based on FPG, PPG and HbA1c values.
Methods
Study Population
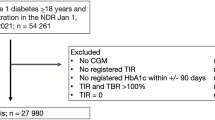
Subjects were enrolled in this study from the Nanjing First Hospital affiliated with Nanjing Medical University. Patients who met the following criteria were studied: (1) patients who were newly diagnosed with T2D or on stable antidiabetic therapy; (2) patients who consented to an OGTT/MMTT before the treatment period; (3) patients who collected CGM profiles within 14 days of OGTT or MMTT test; and (4) patients with CGM data sufficient to calculate TIR.
A total of 399 subjects were included in this study; 195 (49%) of them were newly diagnosed patients with T2D, and the rest (51%) were patients who were stable on at least 6 months antidiabetic therapy without any change. A total of 154 (39%) subjects underwent an OGTT, and 245 (61%) subjects underwent an MMTT. Age, height, weight and medical history were recorded. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2).
This study has used only retrospective data, and therefore ethics committee approval was not required. However, data use in our study was approved by the Research Ethics Committee of Nanjing First Hospital (ky20120928-06) and conducted in compliance with the Declaration of Helsinki and current ethical codes; all patients provided informed consent.
Biochemical Investigations
Blood samples were collected after overnight fasting and 2 h after OGTT or MMTT. Blood glucose levels were measured using the glucose oxidase method with an autoanalyzer (Modular E170, Roche, Mannheim, Germany). HbA1c was measured by high-performance liquid chromatography assay (Bio-Rad Laboratories, Inc., CA, USA).
CGM and Data Processing
CGM was performed in an iPro2 CGM system (Medtronic, MiniMed, USA). These CGM devices continuously measure interstitial glucose levels within a range of 40–400 mg/dL every 5 min, up to a maximum of 288 read counts per day. For each patient, CGM data with a minimum of 70% of possible CGM readings over a day and collected within 14 days of MMTT/OGTT were retained. On average, 3 days of CGM data for each subject were kept for analysis. TIR referred to the time spent in an individual’s target glucose range (70–180 mg/dL [3.9–10 mmol/L]). The minimum and maximum glucose concentrations during each nocturnal and meal-intake (breakfast, lunch, dinner) period were extracted to assess the glucose variability and fluctuations (Supplementary Fig. S1). For the hospitalized management, meals were generally served at 7:00, 11:00 and 17:00, so we considered three meal-intake period (breakfast, lunch, dinner) as 7.00–10.59, 11:00–16.59 and 17:00–21:59, respectively. The minimum and maximum glucose for each period referred to the lowest and highest glucose values during this period. And the nocturnal minimum glucose referred to the lowest glucose value from 22:00 to 06:59.
Prediction Model
The dataset was randomly split into a training set (80%) and a test set (20%). This step was repeated 20 times to generate 20 replicates for the training sets and testing sets. Then, the prediction models were built and tested on these datasets.
Univariate linear regression, multivariate linear regression and random forest regression were used to predict TIR. The univariate linear regression model estimated TIR with HbA1c only, whereas the multivariate linear regression models utilized FPG, PPG and HbA1c to predict TIR. In the random forest regression model, TIR was predicted with FPG, PPG and HbA1c in a nonlinear manner. TIR prediction model based on other variables has been evaluated too, and the results are shown in Supplementary Table 1.
The function lm from the R package stats was used to access the linear regression models. The R package randomForest was used to perform the random forest model. The number of trees in the random forest model was set to 400, and other parameters were kept at the default settings.
Model Evaluation
Pearson’s correlation coefficient (R), mean absolute error (MAE), root mean square error (RMSE) and mean square error (MSE) were used to evaluate the prediction performance of the models. The models were first built on the training sets and then applied on the testing sets. As there were 20 training sets and testing sets, R, MAE, RMSE and MSE were calculated for each of the 20 sets. Student’s t test was used to compare the results between different models to measure the prediction performance. MAE is a measure of the absolute average magnitude of the errors in a set of predictions, without considering their direction. It measures the average difference between the actual and predicted values. MAE is calculated as the average of the absolute differences between the actual and predicted values [3]. RMSE is another measure of the average magnitude of the errors. RMSE is the square root of the mean of the squared differences between the actual and predicted values [4]. MSE is the mean of the squared differences between the actual and predicted values. MSE provides information about the overall prediction performance of the model by measuring the average of the squared differences between the actual and predicted values.
Statistical Analyses
All demographic information is presented as frequencies and percentages for categorical variables and mean ± SD for continuous variables in Table 1. Linear regressions were applied to assess the association of continuous variables. Correlation coefficients and p values for each variable were used to determine the relationship between FPG, PPG, HbA1c and maximum/minimum glucose. The R2 and p values were used to evaluate the linear relationship in regression. All analyses were conducted with R, version 4.1.0.
Results
Individual Trial Characteristics
A total of 399 Chinese patients with T2D were included in this study; 154 (39%) subjects received OGTT to access the glycaemic profile, and 245 (61%) subjects received MMTT to access the glycaemic profile. The median age was 56 years, and a majority of the participants were male. The mean HbA1c was 8.91% (Table 1).
Association Between HbA1c, FPG, PPG and Minimum/Maximum Glucose Values
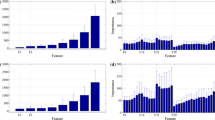
FPG, PPG and HbA1c values are strongly correlated with minimum and maximum glucose concentrations during a day as measured through CGM data (Fig. 1, Supplementary Fig. S2). FPG concentrations were positively correlated with minimum glucose concentrations, particularly with minimum glucose concentrations at breakfast (p < 0.001). PPG concentrations, on the other hand, were the most positively correlated with maximum glucose concentrations, especially at breakfast (p < 0.001), compared with the coefficient of minimum and maximum glucose concentrations at lunch and dinner. The significance level and coefficient of HbA1c values are overall less comparable to those of FPG and PPG.
Association Between FPG, PPG, HbA1c and %TIR Values
Linear regression analysis was used for the investigation of association between HbA1c and %TIR values. As shown in Fig. 2, there was a significant correlation between HbA1c and TIR values (R2 = 0.26, p < 0.001). The correlation was strengthened when FPG and PPG concentrations were added to the multivariate regression analysis (R2 = 0.36, p < 0.001). In multivariate linear regression, FPG and PPG concentrations were incorporated with HbA1c values as arguments. All three arguments are significantly correlated with %TIR values. The coefficient of HbA1c values in multivariate linear regression decreased from − 8.17 to − 2.91.
Linear regression analysis to evaluate the correlation of HbA1c with TIR (left) and multiple variables (HbA1c, FPG, PPG) with TIR (right). R2 for correlation of HbA1c with TIR, 0.2586; R2 for correlation of multiple variables with TIR, 0.3592. TIR time in range, FPG fasting plasma glucose, PPG postprandial plasma glucose
TIR Prediction Model in the General T2D Population
A performance matrix of the prediction model to estimate TIR was constructed to evaluate the performance of the multivariate linear and nonlinear models (FPG, PPG, HbA1c) and the univariate linear model (HbA1c). The results demonstrate that the multivariate nonlinear random forest model (R = 0.79, RMSE = 18.16, MAE = 14.77) outperformed the linear model and the univariate model, and the multivariate linear model (R = 0.73, RMSE = 20.17, MAE = 16.67) was superior to the univariate model (R = 0.62, RMSE = 25.97, MAE = 21.29) in the same training and testing sets (Table 2). It is worth mentioning that the univariate regression model was built upon a previously published formula [31]. The predictions based on testing set among the three models are shown in Fig. 3.
Scatter plot for CGM-derived TIR and predicted TIR between univariate linear regression model (A), multivariate linear regression model (B), random forest regression model (C) in randomly generated testing set. X CGM-derived TIR, Y predicted TIR, TIR time in range, FPG fasting plasma glucose, PPG postprandial plasma glucose, R Pearson’s correlation coefficient, MSE mean square error, MAE mean absolute error, RMSE root mean squared error
TIR Prediction Model in the Newly Diagnosed T2D and Existing T2D Population
The subgroup modelling in the newly diagnosed T2D and existing T2D population displayed an improved prediction performance for random forest model as we stratified and simplified the disease history of the subjects. For newly diagnosed T2D population, the correlation coefficients between the true and predicted values for the random forest regression model were 0.84, and 15.89 and 13.15 in RMSE and MAE, respectively; the correlation coefficients for the multivariate linear model were 0.8, and 17.16 and 14.50 in RMSE and MAE, respectively. For the existing T2D population, the correlation coefficients between the true and predicted values for the random forest regression model were 0.84, and 18.48 and 15.26 in RMSE and MAE, respectively (Tables 3, 4).
Discussion
In this study, we investigated the relationship between three clinically widely used glycaemic parameters (FPG, PPG, HbA1c) and HbA1c values, and the CGM-derived minimum and maximum glucose concentrations in patients with T2D. The results reveal that FPG and PPG concentrations measured by the glucose tolerance test could to some extent reflect glycaemic excursion within a day. We observed the highest correlation coefficient between FPG and minimumbreakfast as well as PPG and maximumbreakfast, which is consistent with the clinical approach, as the measurements of FPG and PPG through MMTT or OGTT are typically performed in the morning time by clinicians. HbA1c is not as sensitive as FPG and PPG to the glycaemic excursion represented by the minimum and maximum glucose during a day. The results are further supported by the correlation analysis between the TIR and those three glycaemic parameters. In the linear regression, a significant correlation between HbA1c and TIR values (R2 = 0.26, p < 0.001) was observed, and the correlation with TIR was further improved by adding FPG and PPG to the model (R2 = 0.36, p < 0.001). All three parameters are significantly correlated with the TIR. The results suggest that the FPG and PPG can also be an indicator of glucose fluctuation in a relatively longer period of time and further demonstrate the potential of FPG and PPG in the estimation of TIR values.
In the light of the results above, we built TIR prediction models based on different parameters involved and algorithms. The results of the TIR prediction models in the same training and testing sets showed that the nonlinear model was superior to the linear model of FPG, PPG and HbA1c, and the multivariate linear model outperformed the univariate linear model. These results are similar to those of previous studies [16, 32], although those studies only evaluated each correlation between FPG, PPG, HbA1c and TIR values separately, while the multivariate relationship has never been considered together.
By incorporating FPG and PPG data into the analysis, we confirmed the limitations of relying solely on HbA1c values to describe and predict TIR. The performance of TIR estimation is significantly improved. The results demonstrate that the estimation of TIR requires a comprehensive understanding of glucose fluctuations beyond that provided by HbA1c values. The superiority of the random forest regression model over the linear model highlights the complex relationship between TIR values and glucose profiles, as TIR measures the diabetes control status in terms of glucose variation and the time window of glycaemic management.
The subgroup analysis shows additional comparisons on segments of the study population, and it demonstrates that there were significant differences in the performances of the prediction model among the newly diagnosed T2D, existing T2D and the general population. The prediction performance in multivariate models is improved in the newly diagnosed T2D population. However, for the existing T2D population, prediction performance on multivariate models was not fully outperformed compared with models is the general population, as the individual errors measured by RMSE, MAE and MSE are slightly raised but the overall coefficient was improved. This means that the background diversity in patients with T2D leads to great differences in glucose profiles; thus, the relationship between TIR and glucose values is different. This has been well demonstrated by a previous study, as their CGM data revealed that patients with different HbA1c values exhibited different glycaemic variations, and patients with higher HbA1c values had larger glycaemic variations. For patients with these characteristics, special therapies aimed at reducing glycaemic variations and prolonging time-to-peak hyperglycaemia are recommended [33]. The establishment of personalized models based on population characteristics is helpful to improve the adaptability and accuracy of the models in different populations. And further confirmed that the correlation analysis of all individuals with a single variable linear model is not enough for TIR estimation.
As discussed, previous studies of CGM metrics and glucose profiles have focused on illustrating the relationship between those variables. It provided the association of the change in HbA1c with TIR elevation and was evaluated through regression analysis. The regression model may not be extended to other datasets for the nature of its purpose. In this study, we evaluated three regression models in the same training and testing sets. The univariate linear regression model for TIR and HbA1c was adopted from a published formula [31]. The model performance evaluation is based on R, RMSE, MAE and MSE of the observed value and predicted value. The results suggest the robustness of the random forest regression model in estimating TIR through FPG, PPG and HbA1c over traditional regression methods.
Although HbA1c remains the gold standard biomarker for long-term glycaemic control, a large percentage of patients with desirable HbA1c control still face the risk of T2D complications, especially when diabetes management focuses on only one metric instead of considering a comprehensive glucose profile [5,6,7,8, 32, 34]. Among patients with the same HbA1c levels, there may be significant differences in their blood glucose fluctuations as reflected by CGM [35], resulting in substantial variability in their TIR [16, 36]. As CGM technology has become widely accepted in the clinical practice of T2D management lately, as a generated parameter from CGM data, TIR has been generally applied in continuous interpretation of glucose data [16, 37] and strongly recommended by various guidelines or consensus worldwide [38,39,40,41]. The American Diabetes Association (ADA)’s Standards of Medical Care in Diabetes—2020 [42] recommended a 70% TIR (70–180 mg/dL) target for optimized T2D management. TIR reveals the glycaemic fluctuations and variability that cannot be easily detected by traditional monitoring methods, which is advantageous for achieving glucose homeostasis and may provide additional information to HbA1c [43]. With this comprehensive glucose biomarker, clinicians can accurately comprehend fluctuations in patients’ glucose levels and evaluate the patterns of their patients’ current lifestyles as well as the efficacy and safety of their current therapeutic method, avoiding hypoglycaemic events and adjusting the treatment programs as needed. However, the use of TIR as an alternative to HbA1c has been debated [44]. The adoption of CGM technology in clinical settings faces several challenges, such as additional cost and time for patients and clinical trials, maintenance and interference from different devices and systems, the requirement of proper training in interpreting ambulatory glucose profile (AGP) reports, and limited availability of the technology, especially for undeveloped regions or for specific patient populations [21, 23, 25]. Accurate and effective assessment of TIR using non-CGM and clinically accessible biomarkers is imperative in clinical practice. By considering the feasibility of the TIR estimation, we aim to provide an alternative approach that may be more accessible and feasible, particularly in resource-limited or rural areas. The glucose tolerance test and HbA1c test are well-established clinical tests that can be performed in various healthcare settings, including those with limited resources. They involve a one-time procedure that does not require continuous wear or specialized equipment. Therefore, in areas where CGM availability is limited, the approach can serve as a valuable tool for estimating TIR and providing insights into glycaemic control.
Our TIR prediction models provide a good fit for external validation with collected multiorigin data. It facilitates clinicians’ assessment of the change in TIR in patients with T2D and the adjustment of therapeutic methods even without CGM devices. However, the unrecognized patient-specific covariates and the uncertainty of disease history may limit the applicability of the models among different populations. A more personalized model may further improve the model performance for patients with different characteristics and disease histories. Random forest regression is a machine learning model that has been adopted by many studies for disease or complication risk prediction [45, 46]. Its strength lies in its ability to handle nonlinear relationships between variables and its robustness to outliers, making it a powerful tool for modelling complex relationships. It also has the ability to handle large datasets, which is useful in TIR prediction models where multiple variables such as FPG, PPG and HbA1c are considered. Additionally, the use of multiple decision trees in the model provides greater stability and reduces the risk of overfitting. The results from this study have shown its potential in the prediction of TIR and have shown its robustness compared with other models. One of the limitations of this study is a limited population of Chinese Han individuals, meaning that the results and performance of the TIR prediction model might not be perfectly generalized to all populations. At the same time, the performance differences in subgroup modelling highlight the potential to enhance performance by incorporating additional disease history information into the model. Additionally, FPG and PPG concentrations were assessed through both OGTT and MMTT, which may introduce variability into the dataset. And the TIR was calculated through a 3-day CGM dataset obtained from the inpatient setting with the Medtronic iPro2 CGM system [47], which does not align with the recent consensus recommendation of at least 10 days of data for predominantly personal use CGM devices [42, 47]. Future studies could optimize the model by incorporating diverse CGM systems with longer monitoring durations, which would help reduce systematic errors and improve the prediction performance of TIR. Besides, the relatively small sample size used in the machine learning model may result in overfitting and affect the representativeness of the training and testing sets; however, this limitation has been partially addressed through cross-validation and the inherent nature of the random forest model. Validation with a more diverse and larger sample size is needed in future work.
Conclusion
Our study highlights the strong correlation between fasting and postprandial glycaemic profiles and the minimum and maximum glucose levels measured by CGM. The important characteristics of glycaemic variation associated with TIR are not appropriately described by HbA1c alone. The TIR prediction model that incorporates FPG, PPG and HbA1c yields significantly improved results compared to the univariate model. Our findings further support the presence of a nonlinear relationship between TIR and these variables, as evidenced by the superior performance of the random forest regression model and the subgroup modelling analysis. The results suggest the presence of an applicable model for TIR estimation. The application of machine learning methods in clinical data modelling setting, especially in the area of chronic disease, provides a new insight into future patients’ disease management.
References
Yapanis M, James S, Craig ME, O’Neal D, Ekinci EI. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J Clin Endocrinol Metab. 2022;107(6):e2221–36.
Cryer PE. Hypoglycemia in diabetes: pathophysiology, prevalence, and prevention. Alexandria: American Diabetes Association; 2016.
Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–44.
Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med. 2021;38(5): e14528.
Uemura F, Okada Y, Torimoto K, Tanaka Y. Association between time in range and postprandial glucose contribution rate in non-insulin-treated type 2 diabetes patients: inverse correlation of time in range with postprandial glucose contribution rate. Diabetes Technol Ther. 2022;24(11):805–13.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Su G, Mi S, Li Z, et al. Prognostic value of early in-hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol. 2013;12:33.
Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–206.
Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–82.
International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org.
Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: evidence from the PREDICTIVE study. Diabetes Res Clin Pract. 2009;86(2):e15–8.
Wright LA, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19(S2):S16–26.
Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care. 2011;34(Suppl 2):S120–7.
Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. 2020;19(1):102.
Majithia AR, Kusiak CM, Armento Lee A, et al. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor-driven virtual diabetes clinic: prospective trial. J Med Internet Res. 2020;22(8):e21778.
Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–26.
Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10(3):853–63.
Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–6.
Huang R, Wang H, Shen Z, et al. Increased glycemic variability evaluated by continuous glucose monitoring is associated with osteoporosis in type 2 diabetic patients. Front Endocrinol (Lausanne). 2022;13: 861131.
Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549–55.
Lanning MS, Tanenbaum ML, Wong JJ, Hoodz KK. Barriers to continuous glucose monitoring in people with type 1 diabetes: clinician perspectives. Diabetes Spectr. 2020;33(4):324–30.
World Bank. A platform of free and open access to global development data by the World Bank. Available from: https://data.worldbank.org/indicator/. Accessed 30 May 2023.
Anhalt H. Limitations of continuous glucose monitor usage. Diabetes Technol Ther. 2016;18(3):115–7.
Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146–56.
Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. 2020;38(5):429–38.
Heinemann L. Continuous glucose monitoring and clinical trials. J Diabetes Sci Technol. 2009;3(4):981–5.
Fox BQ, Benjamin PF, Aqeel A, et al. Continuous glucose monitoring use in clinical trials for on-market diabetes drugs. Clin Diabetes. 2021;39(2):160–6.
CDS. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. 2021;13(4):315–409.
El Malahi A, Van Elsen M, Charleer S, Dirinck E, et al. Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2022;107(2):e570–81.
Zhou J, Mo Y, Li H, et al. Relationship between HbA1c and continuous glucose monitoring in Chinese population: a multicenter study. PLoS ONE. 2013;8(12):e83827.
Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–5.
Liao B, Chen Y, Chigutsa F, Piras de Oliveira C. Fasting and postprandial plasma glucose contribution to glycated haemoglobin and time in range in people with type 2 diabetes on basal and bolus insulin therapy: results from a pooled analysis of insulin lispro clinical trials. Diabetes Obes Metab. 2021;23(7):1571–9.
Li FF, Liu BL, Yan RN, et al. Features of glycemic variations in drug naïve type 2 diabetic patients with different HbA1c values. Sci Rep. 2017;7(1):1583.
Martinez M, Santamarina J, Pavesi A, et al. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1): e002032.
Ajjan RA. How can we realize the clinical benefits of continuous glucose monitoring? Diabetes Technol Ther. 2017;19(S2):S27–36.
Beck RW, Connor CG, Mullen DM, et al. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–9.
Timmons JG, Boyle JG, Petrie JR. Time in range as a research outcome measure. Diabetes Spectr. 2021;34(2):133–8.
ElSayed NA, Aleppo G, Aroda VR, et al. Standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1).
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925–66.
Wilmot EG, Lumb A, Hammond P, et al. Time in range: a best practice guide for UK diabetes healthcare professionals in the context of the COVID-19 global pandemic. Diabet Med. 2021;38(1):e14433.
Aleppo G. Clinical application of time in range and other metrics. Diabetes Spectr. 2021;34(2):109–18.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl. 1):S66–76.
Monnier L, Colette C, Owens D. Application of medium-term metrics for assessing glucose homoeostasis: usefulness, strengths and weaknesses. Diabetes Metab. 2021;47(2):101173.
Heinemann L, Freckmann G, Müller-Wieland D, et al. Critical reappraisal of the time-in-range: alternative or useful addition to glycated hemoglobin? J Diabetes Sci Technol. 2020;14(5):922–7.
Dagliati A, Marini S, Sacchi L, et al. Machine learning methods to predict diabetes complications. J Diabetes Sci Technol. 2018;12(2):295–302.
Dinh A, Miertschin S, Young A, et al. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. 2019;19(1):211.
Medtronic. User Guide of Medtronic iPro2 CGM system. Available from: https://www.medtronicdiabetes.com/download-library/ipro-2. Accessed 30 May 2023.
Acknowledgements
We thank the patients, study coordinators and the members of the endocrinology department of Nanjing First Hospital for their support.
Funding
This study was supported by the Health Science and Technology Development Fund Project of Nanjing Municipal Health Commission (No. ZKX22038). No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Jianhua Ma, and Xiaofei Su contributed to the conception and design of the study. Rui Sun and Yumei Zhang wrote the first draft of the manuscript. Yanli Duan, Bo Ding and Rengna Yan contributed to provide clinical samples and the acquisition of data. Lingge Feng carried out the statistical analysis and model construction of the study. Jianhua Ma and Xiaofei Su corrected and approved the revisions and final version of the manuscript. All authors had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors revised the manuscript critically for important intellectual content.
Disclosures
Lingge Feng is an employee of Hua Medicine. Rui Sun, Yanli Duan, Yumei Zhang, Bo Ding, Rengna Yan, Jianhua Ma and Xiaofei Su have nothing to disclose.
Compliance with Ethics Guidelines
Data use in our study was registered by the registration body approved by WHO, approved by the Ethics Committee of Nanjing First Hospital (ky20120928-06) and all patients provided informed consent in these studies before taking part.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sun, R., Duan, Y., Zhang, Y. et al. Time in Range Estimation in Patients with Type 2 Diabetes is Improved by Incorporating Fasting and Postprandial Glucose Levels. Diabetes Ther 14, 1373–1386 (2023). https://doi.org/10.1007/s13300-023-01432-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01432-2