Abstract
Background
Acute phase hyperglycemia has been associated with increased mortality in patients with acute myocardial infarction (AMI). However, the predictive value of glycemic excursion for adverse outcome in elderly AMI patients is not clear. The aim of this study is to investigate the prognostic value of early in-hospital glycemic excursion and hemoglobin A1c (HbA1c) for one-year major adverse cardiac event (MACE) in elderly patients with AMI.
Methods
We studied 186 elderly AMI patients, whose clinical data were collected and the Global Registry of Acute Coronary Events (GRACE) risk score were calculated on admission. The fluctuations of blood glucose in patients were measured by a continuous glucose monitoring system (CGMS) for 72 hours. Participants were grouped into tertiles of mean amplitude of glycemic excursions (MAGE) and grouped into HbA1c levels (as ≥6.5% or <6.5%). The MACE of patients, including new-onset myocardial infarction, acute heart failure and cardiac death, was documented during one year follow-up. The relationship of MAGE and HbA1c to the incidence of MACE in elderly AMI patients was analyzed.
Results
In all participants, a higher MAGE level was associated with the higher GRACE score (r = 0.335, p < 0.001). The rate of MACE by MAGE tertiles (>3.94 mmol/L, 2.55-3.94 mmol/L or <2.55 mmol/L) was 30.2% vs. 14.8% vs. 8.1%, respectively (p = 0.004); by HbA1c category (≥6.5% vs. <6.5%) was 22.7% vs. 14.4%, respectively (p = 0.148). Elderly AMI patients with a higher MAGE level had a significantly higher cardiac mortality. In multivariable analysis, high MAGE level was significantly associated with incidence of MACE (HR 3.107, 95% CI 1.190-8.117, p = 0.021) even after adjusting for GRACE risk score, but HbA1c was not.
Conclusions
The early in-hospital intraday glycemic excursion may be an important predictor of mortality and MACE even stronger than HbA1c in elderly patients after AMI.
Similar content being viewed by others
Background
Increasing age is considered one of the most significant risk factors for acute myocardial infarction (AMI) [1]. Ageing is also a risk factor that contributes to variance in diabetes risk [2]. Dysglycemia is associated with poor outcomes in AMI patients both with and without diabetes. Chronic glucose dysregulation, as assessed by haemoglobin A1c (HbA1c) levels, is a prognostic factor for mortality in patients with AMI [3, 4]. It is evident that admission hyperglycemia is of independent prognostic value with regard to future adverse cardiovascular events in patients with AMI [5, 6]. Recent studies have showed that glycemic excursion might play an important role in the pathogenesis of atherosclerosis and may be an independent risk factor for cardiovascular complications in diabetics [7, 8]. However, it still remains unclear whether acute glycemic excursion has the important prognostic significance in elderly AMI patients. The purpose of the current study is therefore to investigate the independent prognostic value of glycemic excursion determined by a continuous glucose monitoring system (CGMS) and HbA1c levels in elderly patients with AMI.
Methods
Study population
Consecutive patients admitted to the cardiology department of Beijing An Zhen Hospital of Capital Medical University for AMI between July 2010 and October 2011 were selected. The inclusion criteria were: (i) age ≥ 60 years old; (ii) confirmed admission diagnosis of AMI including ST segment elevated myocardial infarction (STEMI) and non-ST segment elevated myocardial infarction (NSTEMI); (iii) admission glucose < 16.7 mmol/L, and without diabetic ketosis or nonketotic hyperosmolar coma. AMI was defined as acute if the time elapsed between the first symptom and admission was 72 h or less. To enable long-term follow-up and repeated visits to our outpatient clinic, only patients under the age of 80 and living within the hospital’s catchment area were eligible. The exclusion criteria were severe non-cardiac disease with expected survival of less than one year and unwillingness to participate. A patient could only be included once. Data, including information on previous clinical history, cardiovascular risk factors and medication, were collected in hospital. Type 2 diabetes mellitus (T2DM) was diagnosed according to the American Diabetes Association criteria or the use of insulin or glucose-lowering medication. The estimated glomerular filtration rate (eGFR) value was calculated by MDRD equation [9]. The Global Registry of Acute Coronary Events (GRACE) risk score were calculated as admission [10], which is recommended by the National Institute for Health and Clinical Excellence (NICE) to assess risk in patients with ACS. Patients were categorized according to tertiles of the mean amplitude of glycemic excursions (MAGE) level (<2.55 mmol/L, 2.55-3.94 mmol/L and >3.94 mmol/L), and according to HbA1c (<6.5% and ≥6.5%) [11]. The study protocol was approved beforehand by the Medical Ethics Committee of Beijing An Zhen Hospital of Capital Medical University and the procedures followed were in accordance with the institutional guidelines. The study complied with the declaration of Helsinki and informed consent was obtained from all patients.
Continuous glucose monitoring
All patients were equipped with CGMS (Medtronic MiniMed, USA), and were monitored for 72 consecutive hours after admission. A CGMS sensor was inserted into the subcutaneous abdominal fat tissue, calibrated according to the standard Medtronic MiniMed operating guidelines. During CGMS monitoring, patients checked their blood glucose level with a self-monitoring of blood glucose (SMBG) device (Medisafe Mini, Terumo, Japan) at least 4 times per day. Then, they entered the SMBG data and time of each meal into the CGMS. After monitoring for 72 hours, the recorded data were downloaded into a personal computer for analysis of the glucose profile and glycemic excursion parameters with MiniMed Solutions software. After downloading the recorded data, the intermediate 48 hours of recording was analyzed to avoid bias due to insertion and removal of the CGMS or insufficient stability of the monitoring system, and MAGE was caculated from the first 24 hours of them. Since measurable range of glucose by CGMS was mechanically limited from 2.2 to 22.2 mmol/L, the case showing the data out of this range was excluded from the study. The MAGE was calculated by measuring the arithmetic mean of the differences between consecutive peaks and nadirs, provided that the differences are greater than one standard deviation (SD) of the mean glucose value [12]. Patients would maintain anti-hyperglycemic therapy as usual and be avoided glucose infusion during CGMS monitoring period. Otherwise, the patient would be excluded from the study.
Biochemical investigations
Blood samples were collected after overnight fasting and stored at −70°C prior to analysis. Serum creatinine, total cholesterol (TC) and triglyceride (TG) levels were measured by automatic biochemical analyzer (Hitachi 747, Tokyo, Japan). Serum concentration of hemoglobin A1c (HbA1c) was determined by high-performance liquid chromatographic method using automatic HbA1c analyzer (Tosoh HLC-723 G7, Japan).
Follow-up
Patients were followed up prospectively for about 1 year. During follow-up period, incidences of major adverse cardiac event (MACE) were registered, including new-onset myocardial infarction, acute heart failure and cardiac death. All MACE data were adjudicated by an experienced cardiovascular physician blinded to clinical details and outcomes.
Statistical analysis
All statistical analyses were performed by using SPSS for Windows 13.0 (SPSS Inc, Chicago, IL, USA). Data are presented as frequencies and percentages for categorical variables and mean ± SD for continuous variables, unless otherwise indicated. Differences between two groups were assessed by using the Chi-square and unpaired t-tests. Correlation between continuous variables was determined by Spearman correlation coefficients. MAGE was included as a continuous and as a categorized (<2.55 mmol/L, 2.55-3.94 mmol/L and >3.94 mmol/L) variable. HbA1c level was also included as a continuous and categorized (<6.5% and ≥6.5%) variable. Kaplan-Meier survival curve analysis was used to represent the proportional risk of MACE for the MAGE and HbA1c values, and the log-rank test was performed to assess differences between high MAGE level and low MAGE level, and high HbA1c level and low HbA1c level. Cox proportional-hazards regression models were used to estimate hazard ratios of clinical variables with regard to MACE. A value of p < 0.05 was considered statistically significant.
Results
Baseline characteristics
During the study period, 200 elderly AMI patients were enrolled. 186 patients with complete data were included in the final analysis (8 patients were removed from study for severe dysglycemia during CGMS monitoring period; 6 patients were excluded from study for incomplete follow-up data). Mean age was 67.0 ± 5.7 years, 60.4% were male and 54.3% had diabetes. Participants were treated conservatively (9.1%), with PCI (80.1%) or with CABG (10.8%). HbA1c was < 6.5% in 111 (59.7%), ≥ 6.5% in 75 (40.3%). The GRACE risk score ranged from 78 to 235 with a mean of 148 ± 36. Baseline characteristics of patient groups based on MAGE and HbA1c are shown in Table 1 and 2, respectively. The correlation of GRACE score with MAGE or HbA1c was significant (Spearman r = 0.335, p < 0.001; r = 0.188, p = 0.010).
Incidences of MACE
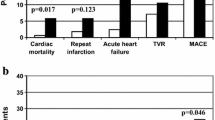
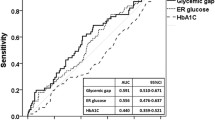
At the end of 1-year follow-up, 10 patients had died (5.4%) for cardiac causes, 13 patients had new-onset myocardial infarction (7.0%), and 10 patients had acute heart failure (5.4%). As expected, elderly AMI patients with MAGE level >3.94 mmol/L had significantly higher incidence of MACE compared with elderly AMI patients with MAGE level from 2.55 mmol/L to 3.94 mmol/L, or < 2.55 mmol/L (30.2% vs. 14.8% vs. 8.1%, p = 0.004). No significant rates of adverse cardiovascular events were observed between patients with HbA1c level ≥6.5% and patients with HbA1c level < 6.5% (22.7% vs. 14.4%, p = 0.148). Elderly AMI patients with a higher MAGE level had a significantly higher cardiac mortality compared with elderly AMI patients with lower MAGE levels (11.1% vs. 1.6% vs. 3.2%, p = 0.043) (Figure 1). No significant differences in rates of adverse cardiovascular events were observed between elderly AMI patients with high HbA1c level and patients with low HbA1c level (Figure 2). Kaplan-Meier survival curves for patient groups by MAGE are shown in Figure 3; those for HbA1c in Figure 4.
Multivariable analysis
To investigate the associations between MAGE, HbA1c level and incidences of MACE with respect to baseline characteristics, we used multivariable analysis. Include variables were: age, gender, and all variables that were significantly different between MAGE or HbA1c categories [current smoking, diabetes, previous coronary artery disease (CAD), heart rate, eGFR, anti-hyperglycemic agents, fasting blood glucose, the STEMI presentation, left ventricular ejection fraction (LVEF, categorized into <50% = 1 and ≥50% = 0) and diuretics]. The independent predictors of MACE were: age (HR 1.624, 95% CI 1.036-2.548, p = 0.035), previous CAD (HR 2.907, 95% CI 1.227-6.896, p =0.015), LVEF (HR 2.611, 95% CI 1.107-6.135, p = 0.028) and MAGE (HR 3.131, 95% CI 1.422-6.710, p = 0.014). HbA1c level was not significantly associated with MACE (HR 1.522, 95% CI 0.841-2.757, p = 0.165). After adjustment for the GRACE score, MAGE was found to be also associated with incidences of MACE (HR 3.107, 95% CI 1.190-8.117, p = 0.021), but HbA1c was not (HR 1.438, 95% CI 0.619-5.464, p = 0.273).
Discussion
Hyperglycemia on admission is common in patients with AMI, and it is a powerful predictor of survival and increased risk of MACE in patients both with and without T2DM [3, 5, 6]. HbA1c is a convenient marker of long-term glycometabolic status. Elevated HbA1c is associated with increased cardiovascular risk in patients. However, patients with similar mean glucose or HbA1c levels can have markedly different glycemic excursions [13]. Acute glucose fluctuations seem to have more deleterious effects than sustained hyperglycemia in the development of cardiovascular complications as glucose both upward and downward changes activate the oxidative stress [14, 15]. We investigated the association between glycemic excursion, HbA1c and one-year MACE in elderly patients with AMI. Our study demonstrated that elevated MAGE was a strong and independent predictor of increased risk of MACE in elderly patients with AMI, but HbA1c was not.
There were major differences in baseline characteristics according to MAGE or HbA1c level. Patients with higher MAGE or HbA1c level had more cardiovascular risk factors, such as older age, diabetes, heart failure or renal insufficiency. There was also a clear correlation between GRACE risk scores and MAGE or HbA1c. The results indicate that elderly AMI patients with worse glycometabolic disorders may be associated with poorer outcomes.
More and more evidences show that glycemic variability may be an important parameter used to resolve potential clinical problems in diabetes. It is reported that postchallenge glucose excursion is independently related to carotid intima-media thickness and may contribute to the development of atherosclerosis in individuals with T2DM independent of other risk factors [7, 16]. In our previous study, we found that glycemic variability is an important contributing factor in the severity of coronary artery disease, which is independent of the average level of blood glucose [8]. The Verona Diabetes study reported that fasting glycemic variability is an independent predictor of mortality in T2DM patients [17]. Some studies concluded that glycemic excursion was a significant predictor of mortality in critically ill patients independently from mean glucose level and severity of illness [18, 19]. In the present study, patients with a higher MAGE level have higher GRACE risk scores. After 1-year follow-up, a significantly higher incidence of MACE and cardiac mortality were found in those patients. The results indicate that high glucose fluctuations may be associated with the risk of future adverse cardiovascular events in patients with AMI. Multivariable analysis disclosed that in the elderly AMI population, MAGE was an independent predictor of MACE, even after adjusting for GRACE risk score, but HbA1c was not.
Acute hyperglycaemia is a common acute adrenergic signal of stress and is present in myocardial infarction, whereas increased catecholamine levels result in decreased insulin secretion and increased insulin resistance [20]. Although stress-induced hyperglycaemia can partly explain the relation between admission glycemic variability and outcomes, glycemic excursion itself can also be harmful. Ceriello et al. reported that intermittent hyperglycaemia induced a higher degree of apoptosis in endothelial cells than chronic hyperglycaemia [14]. Quagliaro et al. showed that the apoptosis of endothelial cells exposed to intermittent high glucose may be related to a reactive oxygen species (ROS) overproduction, through protein kinase C (PKC)-dependent activation of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase [21]. Glycemic excursion may also be an important mediator in inflammatory responses. In vitro studies indicate that glucose fluctuations can activate nuclear factor-κB and PKC pathway, leading to a greater expression of the adhesion molecules and excess formation of advanced glycation end-products than stable high glucose [22]. Moreover, severe glycemic disorders may adversely affect sympathetic dysfunction which is associated with mortality and morbidity of cardiovascular disease [23].
Although both HbA1c and glycemic excursion may be associated with adverse prognosis, our study show that increased MAGE is more important. In our analysis, the less clear association between HbA1c and MACE could be due to a limited number of patients with a relatively short follow-up in present study. Increased HbA1c represents long-term glucose regulation, whereas elevated glycemic excursion is not only a symptom of glucose dysregulation, but also of stress and general poor health. Carmen Wong et al. found cortisol level is correlated with acute hyperglycaemia in patients with AMI [24]. There is a clear association to be found between HbA1c and long-term outcome in AMI patients after 3.3 years follow-up [25]. These differences may relate to length of follow-up. HbA1c may have limited predictive power for short-term prognosis in patients with AMI, but its association with long-term prognosis may be stronger.
There is still an extensive debate about glycemic excursion as a risk factor for cardiovascular complications independent of HbA1c[26, 27]. Siegelaar et al. performed reanalysis of the data of the HEART2D, which shows that targeting post-prandial glucose decreased intraday glycemic excursion would not be beneficial in reducing adverse cardiovascular events in AMI patients [28]. However, the HEART2D was not designed to determine the impact of glycemic excursion on the risk of MACE, and the MAGE and SD levels were not found significantly different between two contrasting groups in the study. In addition, the method of calculating glycemic excursion from self-measured blood glucose profiles may be not very accurate. Overall, more well-designed studies are needed to investigate whether glycemic excursion will play an important role in the prognosis of AMI.
Study limitations
The sample size was relatively small, so that comparisons of some subgroups might lack power to detect significant differences for selected variables. For lack of microvascular complications data, we didn’t include those risk factors in analysis. Although we had maintained the patients’ anti-hyperglycemic therapy as usual and avoided glucose infusion during CGMS monitoring period, some factors, such as different diets, physical and emotional stress etc., which may affect glucose fluctuations couldn’t be all prevented. In addition, tests to detect diabetes were not routinely done, so some cases of diabetes may have been missed. However, if the observed relation between glycemic excursion and MACE was due to undiagnosed diabetes, one would have expected a more distinct association between HbA1c and outcomes.
Conclusions
In elderly patients, early in-hospital MAGE may be an important predictor of mortality and MACE after AMI even stronger than HbA1c. The results of this study further support the view that glycemic excursion should be one of the targets of treatment for the glycemic disorders encountered in AMI patients. Further studies are needed to determine if pharmacologic therapy aimed at controlling glucose excursion in AMI would be beneficial in prognosis of this high-risk patient population.
References
White HD, Aylward PE, Huang Z, Dalby AJ, Weaver WD, Barvik S, Marin-Neto JA, Murin J, Nordlander RO, van Gilst WH, Zannad F, McMurray JJ, Califf RM, Pfeffer MA, VALIANT Investigators: Mortality and morbidity remain high despite captopril and/or valsartan therapy in elderly patients with left ventricular systolic dysfunction, heart failure, or both after acute myocardial infarction: results from the valsartan in acute myocardial infarction trial (VALIANT). Circulation. 2005, 112: 3391-3399. 10.1161/CIRCULATIONAHA.105.551143.
Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, Teixeira AM, Veríssimo MT: Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol. 2012, 11: 13-10.1186/1475-2840-11-13.
Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, Kilic ID: The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol. 2008, 24: 375-378. 10.1016/S0828-282X(08)70600-7.
Wahsb NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL, ICONS Investigators: Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era?. J Am Coll Cardiol. 2002, 40: 1748-1754. 10.1016/S0735-1097(02)02483-X.
Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, Visser FC: Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004, 164: 982-988. 10.1001/archinte.164.9.982.
Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, Krumholz HM: Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005, 111: 3078-3086. 10.1161/CIRCULATIONAHA.104.517839.
Hu Y, Liu W, Huang R, Zhang X: Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis. 2010, 210: 302-306. 10.1016/j.atherosclerosis.2009.11.015.
Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, Zhou Y, Ma C: Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011, 10: 19-10.1186/1475-2840-10-19.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999, 130: 461-470.
Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA, Global Registry of Acute Coronary Events Investigators: Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003, 163: 2345-2353. 10.1001/archinte.163.19.2345.
American Diabetes Association: Standards of medical care in diabetes-2010. Diabetes Care. 2010, 33 (Suppl 1): S11-S61.
Monnier L, Colette C, Owens DR: Glycemic variability: The third component of the dysglycemia in diabetes. Is it important? How to measure it?. J Diabetes Sci Technol. 2008, 2: 1094-1100.
Monnier L, Colette C: Glycemic variability: should we and can we prevent it?. Diabetes Care. 2008, 31 (Suppl 2): S150-S154.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D: Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008, 57: 1349-1354. 10.2337/db08-0063.
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fuctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006, 295: 1681-1687. 10.1001/jama.295.14.1681.
Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M: Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000, 23: 1830-1834. 10.2337/diacare.23.12.1830.
Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, Verlato G: Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona diabetes study. Diabetes Care. 2000, 23: 45-50. 10.2337/diacare.23.1.45.
Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM, May AK: Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008, 74: 679-685.
Krinsley JS: Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008, 36: 3008-3013. 10.1097/CCM.0b013e31818b38d2.
Takada JY, Ramos RB, Roza LC, Avakian SD, Ramires JA, Mansur Ade P: In-hospital death in acute coronary syndrome was related to admission glucose in men but not in women. Cardiovasc Diabetol. 2012, 11: 47-10.1186/1475-2840-11-47.
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A: Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003, 52: 2795-2804. 10.2337/diabetes.52.11.2795.
Azuma K, Kawamori R, Toyofuku Y, Kitahara Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M, Watada H: Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006, 26: 2275-2280. 10.1161/01.ATV.0000239488.05069.03.
Takei Y, Tomiyama H, Tanaka N, Yamashina A: Close relationship between sympathetic activation and coronary microvascular dysfunction during acute hyperglycemia in subjects with atherosclerotic risk factors. Circ J. 2007, 71: 202-206. 10.1253/circj.71.202.
Carmen Wong KY, Wong V, Ho JT, Torpy DJ, McLean M, Cheung NW: High cortisol levels in hyperglycaemic myocardial infarct patients signify stress hyperglycaemia and predict subsequent normalization of glucose tolerance. Clin Endocrinol (Oxf). 2010, 72: 189-195. 10.1111/j.1365-2265.2009.03654.x.
Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ, Zijlstra F, van’t Hof AW: Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011, 124: 704-711. 10.1161/CIRCULATIONAHA.110.985911.
Kilpatrick ES, Rigby AS, Atkin SL: For debate. Glucose variability and diabetes complication risk: we need to know the answer. Diabet Med. 2010, 27: 868-871.
Lipska KJ, Venkitachalam L, Gosch K, Kovatchev B, Van den Berghe G, Meyfroidt G, Jones PG, Inzucchi SE, Spertus JA, DeVries JH, Kosiborod M: Glucose variability and mortality in patients hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012, 5: 550-557. 10.1161/CIRCOUTCOMES.111.963298.
Siegelaar SE, Kerr L, Jacober SJ, Devries JH: A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. 2011, 34: 855-857. 10.2337/dc10-1684.
Acknowledgements
This work was supported by a key grant from Beijing Health Special Foundation (JING 09–08).
Funding
This study was supported by a key grant from Beijing Health Special Foundation (No. JING 09–08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GS participated in the design of the study, participated in the exercise protocols, performed the statistical analysis and drafted the manuscript. SHM and HT participated in the design of the study and drafted the manuscript. ZL, HXY and HZ participated in the exercise protocols. All authors approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Su, G., Mi, Sh., Li, Z. et al. Prognostic value of early in-hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol 12, 33 (2013). https://doi.org/10.1186/1475-2840-12-33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-12-33