Abstract
Introduction
The aim of this study was to evaluate the stability and accuracy of glucose measurements determined using the metabolic heat conformation (MHC)-based non-invasive glucometer in a multicentre, self-controlled clinical trial. This device is the first to obtain a medical device registration certificate awarded by the National Medical Products Administration of China (NMPA).
Methods
The multicentre clinical study was conducted at three sites and enrolled 200 subjects whose glucose was measured with a non-invasive glucometer (the Contour Plus blood glucose monitoring system) and by venous plasma glucose (VPG) measurements, in a fasted state and at 2 and 4 h after meals.
Results
Based on both the non-invasive and VPG measurements, 93.9% (95% confidence interval 91.7–95.6%) of the blood glucose (BG) values fell within consensus error grid (CEG) zones A + B. The measurements obtained in a fasted state and at 2 h after meals were more accurate, with 99.0% and 97.0% of the BG values, respectively, falling within zones A + B. Compared to those subjects who received insulin, the proportion of values in zones A + B and the correlation coefficients were 3.1% and 0.0596 higher, respectively. The accuracy of the non-invasive glucometer was influenced by the level of insulin resistance calculated by the homeostatic model assessment method, which had a correlation coefficient with the mean absolute relative difference of − 0.1588 (P = 0.0001).
Conclusion
The MHC-based non-invasive glucometer assessed in the present study demonstrates generally high stability and accuracy in the glucose monitoring of people with diabetes. The calculation model needs to be further explored and optimised for patients with different diabetes subtypes, levels of insulin resistance and insulin secretion capacity.
Clinical trial registry number
ChiCTR1900020523.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The non-invasive blood glucose (BG) monitoring (NGM) meter based on the metabolic heat conformation (MHC) method was used to measure BG levels in the fasting and postprandial states (2 and 4 h after meal) in 200 people with diabetes (PWD), and its monitoring accuracy was found to reach clinical acceptable comparison results. |
In accordance with the EN ISO 15197:2013 standard, we used the homeostatic model assessment method to evaluate the clinical accuracy, stability and indicated population of the NGM meter in PWD. |
What was learned from the study? |
Using multiple regression analysis, we analysed the impact of different factors on BG measurement at different time points. |
Under the premise of calibrating information collected from PWD in a non-invasive manner, we explored the performance of the NGM technology based on the MHC method during BG monitoring at different time points. |
Under the premise of using fasting FG as the calibration measurement, fasting C-peptide is an independent factor affecting the accuracy of NGM both in the fasting and in postprandial state. |
Introduction
In recent years, the authors of several epidemiological investigations have reported that the prevalence of diabetes in adults of mainland China is remaining stable at around 10–11% [1]. In mainland China, the increasing number of patients with diabetes as well as difficulties in patient management are accompanied by the negative effects of poor disease management [2]. Poor disease management leads to a significant increase in the risk of chronic complications, while it is well known the implementation of effective disease management can greatly improve the prognosis and reduce long-term costs [3]. Self-monitoring of blood glucose (BG) is crucial for evaluating the effect of daily disease management and for guiding treatment, with recommendations provided by many international guidelines [4, 5]. Particularly for patients who receive insulin therapy, self-monitoring of BG can improve metabolic control and have a beneficial effect on the progression of diabetes-related complications.
In addition to the traditional capillary BG monitoring methods, continuous glucose monitoring (CGM) technology has been developed in recent years, and its accuracy and safety have been recognised [6, 7]. At the same time, guidelines also include the concept of ‘time in range’ based on CGM as a new index to evaluate a patient’s BG control [8, 9]. Although CGM is a minimally invasive method, it still belongs to the category of invasive monitoring techniques. Therefore, it is impossible to avoid a potential reduction in compliance due to patient concerns about its invasive nature.
Non-invasive glucose measurement methods are ideal alternatives for injury- and pain-free BG monitoring [10]. Electrochemical [10,11,12,13] and optical [14,15,16] approaches have received the most attention. Many non-invasive methods are currently under study. Several studies have reported that these methods have a high accuracy, although the sample size in most of these studies has been small (< 40 subjects) [8, 11, 17,18,19,20], with the exception of one study that included 92 subjects [13]. Therefore, the actual clinical performance of these methods cannot yet be assessed accurately.
According to the metabolic heat conformation (MHC) theory, the BG level can be estimated as a function of metabolic heat and oxygen supply at rest [21,22,23]. An non-invasive BG monitoring (NGM) meter has been developed based on this theory, with the device integrating the sensor with a fingertip probe, which connects to a mobile flat-panel display, thus allowing convenient glucose measurements and self-management. The National Medical Products Administration of China (NMPA) awarded this meter a medical device registration certificate in 2019 (Certificate No. NDRS2019307060).
In the present multicentre, self-controlled study, we enrolled 200 subjects, with the aim to compare the results of MHC-based non-invasive glucose measurements with venous plasma glucose (VPG) and fingerstick measurements. We evaluated the clinical accuracy and stability of the NGM and the patient population in people with diabetes according to the EN ISO 15197:2013 standard [24], using subgroup analysis, physiological homeostatic model assessment (HOMA) [25] and multivariate analysis of non-invasive BG monitoring.
Methods
Clinical Trial Design
This multicentre, open-label, self-controlled clinical study was registered in the Chinese Clinical Trial Registry (Registration number ChiCTR1900020523). It was conducted simultaneously at the Peking University First Hospital, the China Meitan General Hospital and the Peking Union Medical College Hospital. The study protocol was approved by the Ethics Review Committee of Peking University First Hospital (ethics approval number: [(2018] Device Registration No.10); the Ethics Review Committee of China Meitan General Hospital (ethics approval number: G18-11); and the Ethics Review Committee of Peking Union Medical College Hospital (ethics approval number: HS2018078). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all individual participants included in the study and their legal guardians. The list of exclusion criteria of subjects are shown in Electronic Supplementary Material (ESM) Table S1 and the list of participating investigators are shown in ESM Table S2. Each participant received a screening examination after they provided informed consent. The screening comprised the collection of data on demographics, medical history and medication history, as well as a physical examination, including measurements of height and weight, determination of vital signs and results of clinical laboratory tests (including haematology, serum chemistry and thyroid stimulating hormone [TSH] levels).
On the following experimental day, the BG level of eligible subjects was measured by using three methods: (1) the MHC-based NGM system (NIGA01; Global Health Ark Medical Technology Co., Ltd. Beijing, China); (2) the Contour Plus Blood Glucose Monitoring System (Bayer AG, Ludwigshafen, Germany); and (3) the VPG analyser based on the hexokinase method. Blood for measurements was drawn when the individual was in a fasting state (fasting glucose test) and at 2 and 4 h postprandial (postprandia glucose test). One meal serving contained 55 g carbohydrates, 7 g protein and 0 g fat, which was eaten within 15 min. Before the meal, non-invasive data were collected on the index finger using the NGM, immediately followed by a fingerstick capillary BG measurement with the Contour Plus system; subsequently, two tubes of 2 mL of blood collected from the upper arm vein were used for VPG measurement and serum insulin and C-peptide measurement, respectively. At 2 h (± 5 min) and 4 h (± 5 min) after the meal, the same glucose measurements were performed in the same order and completed within 5 min. Measurements with the NGM and Contour Plus system were performed on the same hand, while venous blood was collected from either arm. The trial flow chart is shown in Fig. 1.
Participants
The subjects included in the study conformed to all of the following inclusion criteria: (1) agreement to participate in the study and signing the informed consent form (ICF); (2) age between 10 and 80 years (inclusive); and diagnosis of diabetes mellitus or impaired glucose regulation as per the revised diagnostic criteria and classification 1999 by the World Health Organization Expert Committee on Diabetes Mellitus (https://apps.who.int/iris/handle/10665/66040.
Subjects who met any of the exclusion criteria (ESM Table S2) were not eligible for this study.
A total of 210 subjects from the three study sites signed the ICF and attended the screening, of which ten subjects did not meet the inclusion criteria and were excluded from the study. The remaining 200 subjects were enrolled in the study, of whom 190 people had type 2 diabetes, seven had type 1 diabetes and three had impaired glucose regulation. Of these 200 subjects, 195 (97.5%) completed the study. Of the five (2.5%) subjects who dropped out before the end of the study period, four had type 2 diabetes, three dropped out after glucose measurements, one was medically unstable, one was removed from the study early due to hypoglycemia and one was removed from the study early at the discretion of the investigator. The demographic data on the participants are summarised in Table 1.
NGM Test Method
The MHC method is based on the characteristics of human BG metabolism in the resting or equilibrium state and enables non-invasive BG detection by measuring physiological features such as metabolic heat and oxygen consumption [21]. This method was used as described in a previous single-centre study [22]. Metabolic heat is a function of BG concentration and oxygen supply, which in turn is determined by oxygen saturation, haemoglobin concentration and blood flow to local tissue; consequently, the BG concentration is considered to be a function of metabolic heat production, oxygen saturation, haemoglobin concentration and blood flow. Other physiological features, such as heart rate and finger pulse wave, are also considered in the model, which can be expressed as
where H is metabolic heat, Qf is blood flow, SpO2 is oxygen saturation, Hb is the haemoglobin concentration and HR is the heart rate.
The MHC-based device comprises a finger sensor probe that measures the physiological features included in Eq. 1 and a flat-panel display, as shown in Fig. 2a. Metabolic heat (H) is mainly exchanged with the environment in the form of radiation and convection; both forms of heat are estimated by using human body temperature and environmental temperature, and the effect of evaporative heat loss can be compensated by human body humidity and environmental humidity. Thus, H characteristics include four variables, namely fingertip temperature and humidity and ambient temperature and humidity, which are detected via infrared radiation sensors and humidity sensors. SpO2, Hb and HR are calculated by absorption spectra of multi-wavelength visible–near infrared light sensors at 660, 730, 800 and 940 nm. Qf mainly depends on the local blood flow rate, which is detected by using a thermal diffusion method. The rate of temperature change on the rod is detected by using the built-in thermal conductivity rod to estimate the blood flow rate [23].
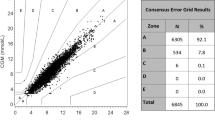
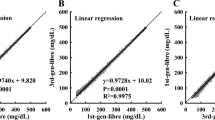
Results of non-invasive measurements. a MHC-based NGM. b CEG analysis. The X-axis represents VPG measurements, and the Y-axis represents non-invasive glucose measurements. The blue, green, pink and red dots represent paired values in CEG zone A, B, C and D, respectively. c Bland–Altman analysis. The X-axis represents the mean of paired NGM readings and VPG measurements, and the Y-axis represents the difference between paired NGM readings and VPG measurements. d Linear-regression analysis. The X-axis represents NGM readings, and the Y-axis represents VPG measurements. CEG Consensus error grid, MHC metabolic heat conformation, NGM non-invasive blood glucose monitoring, SD standard deviation
When used for the first time, a user account needs to be created, during which time the basic user information is entered, such as age, height, weight and type of diabetes. Before measurements, users select the BG measurement feature by following the prompts on the screen. After 10 s of self-inspection, the device instructs the user to attach the sensor probe to a finger within 15 s, and to maintain the chosen posture until the end of the measurements, which lasts for about 1 min. Users are required to avoid washing their hands before the measurement and to keep their finger still.
Data Analysis
The point accuracy of the NGM was evaluated by comparing the NGM measurements with VPG and fingerstick capillary whole BG measurements.
Steady-State HOMA
The homeostatic model assessment (HOMA) with fasting plasma glucose (FPG) and insulin/C-peptide (CP) as inputs has been used extensively for clinic applications, especially to evaluate the level of insulin resistance (HOMAIR) and β cell function (HOMAβ) of patients with diabetes (PWD). We evaluated insulin resistance by measuring the C-peptide level, considering that determination of the C-peptide level is not affected by exogenous insulin level. The mathematical estimation model is shown as Eqs. 2 and 3, wherein FCP denotes the fasting C-peptide concentration (mU/L) and FPG is the fasting plasma glucose (mmol/L).
These two indexes [25, 26] were obtained by using the HOMA (CP) model to evaluate how different levels of insulin resistance and islet β cell function could affect the accuracy.
Accuracy Evaluation
The ISO 15197:2013 standard specifies the performance requirements for in vitro BG monitoring systems measuring the BG concentration in capillary blood samples [24]. This standard was used as the primary evaluation of the NGM readings. The results were analysed by using a two-tailed t-test to obtain the 95% confidence interval (CI). Secondary evaluation measures included the mean absolute relative difference (MARD), Bland–Altman analysis (using the difference between paired BG values as the Y-axis, the mean of paired BG values as the X-axis and the mean difference as the line of means and mean difference ± 1.96 standard deviations [SD] as the limits of agreement) and regression analysis (assessing the correlation between paired BG values by estimating the slope of the regression line and Pearson correlation coefficient for two measurement methods).
Statistical Methods
Multivariate regression analysis was processed using SPSS version 22.0 software (IBM SPSS, Armonk, NY, USA) to represent the measurement data. The independent sample t-test was used to test the inter-group data, and the counting data were expressed by the Chi-squared (χ2) test. The factors influencing the accuracy of non-invasive BG detection were tested by multivariate regression analysis. A P < 0.05 indicated statistical significance.
Results
Non-invasive Measurements
Validity analysis was performed for 589 paired BG values. As shown in Fig. 2b, 93.9% (95% CI 91.7–95.6%) of the paired NGM readings and VPG values fell in consensus error grid (CEG) zones A + B, 320 readings (54.3%, 95% CI 45.7–53.8%) fell in zone A and no readings fell in zone E. The MARD between non-invasive and VPG measurements was 27.02% ± 17.95% (95% CI 24.61–29.44%) (see ESM for subgroup analysis results). According to the results of the agreement test (Fig. 2c), 554 (94.1%) paired BG values showed a difference within the limits of agreement. The regression coefficient of the paired BG values was 0.8289 and the Pearson correlation coefficient was 0.6841 (P < 0.0001), as shown in Fig. 2d.
In total, 94.4% of the paired NGM readings and fingerstick capillary BG measurements fell in CEG zones A + B and 54.7% fell in zone A. The MARD was 26.16% ± 16.25% (95% CI 23.88–28.43). In this study, 93.0% of paired BG values showed a difference within the limits of agreement. The regression coefficient of the paired BG values was 0.7864 and the Pearson correlation coefficient was 0.6627 (P < 0.0001). The comparisons between non-invasive and VPG measurements were similar to those between non-invasive and fingerstick measurements, as shown in ESM Figs. S1–S3.
Classification by BG Measurement Time Points
Non-invasive Measurements
The results of CEG analysis and the correlation coefficients between non-invasive and VPG values obtained in the fasting state and 2 and 4 h after a meal are shown in Table 2. In the fasting state, the NGM readings and VPG values exhibited a good correlation, with a higher percentage of values falling in CEG zone A. As shown in Fig. 3, the percentage of paired readings that fell in CEG zones A + B was similar in the fasting state and at 2 h after a meal (99.0 vs. 97.0%), and was the lowest at 4 h after a meal (85.6%). The NGM readings showed greater clinical accuracy for FPG and plasma glucose 2 h after a meal; this ability could satisfy the demands of patients with diabetes to self-monitor their BG and manage their disease.
Multivariate Analysis of Non-invasive BG Monitoring
Based on the subgroup analyses of glucose measurements at different time points, the fasting state had higher linearity than the other two time periods. We next analysed how various factors affected the glucose measurements at the different time points. We first evaluated the accuracy of non-invasive BG monitoring: non-invasive BG and VPG measurements were performed for participants in the fasting state and at 2 and 4 h postprandial for 197 subjects. The CEG was plotted and the measurements falling in zone A were deemed ‘good’ and included into the ‘Good Group’, while the measurements falling outside zone A were deemed ‘general’ and included into the ‘General Group’. We then considered gender, age, body mass index (BMI), disease course, fasting insulin, fasting C-peptide, HOMAβ (CP), HOMAIR (CP), VPG values and non-invasive measurements. Finally, we used the Chi-square test to identify those variables that were significantly different in the analysis and included them in the multivariate regression model.
In the multivariate regression model, the difference between the non-invasive and VPG measurements was applied to characterise the accuracy of non-invasive BG monitoring and used as the dependent variable in the model. This approach allowed us to obtain the independent factors that influence the accuracy of non-invasive BG monitoring.
When the CEG was plotted in the fasting state, the measurements of 121 of 197 subjects fell into zone A, with the remaining measurements falling outside of zone A. Based on the Chi-square test, six variables were significantly different: fasting insulin (χ2 = 185.890, P = 0.001), fasting C-peptide (χ2 = 147.796, P = 0.003), HOMAβ (CP) (χ2 = 187.756, P = 0.002), HOMAIR (CP) (χ2 = 187.756, P = 0.002), the fasting VPG level (χ2 = 156.556, P = 0.004) and BMI (χ2 = 108.235, P = 0.010). These factors were used as independent variables in the multivariate regression, as shown in Table 3. Multivariate regression analysis revealed that these factors did not significantly influence the accuracy of non-invasive measurements.
When the CEG was plotted 2 h after a meal, the measurements of 126 of 197 subjects fell in zone A; the remaining measurements fell outside of zone A. Based on the Chi-square test, six variables were significantly different: fasting insulin (χ2 = 186.309, P = 0.002), fasting C-peptide (χ2 = 146.392, P = 0.007), HOMAβ (CP) (χ2 = 189.828, P = 0.003), HOMAIR (CP) (χ2 = 189.828, P = 0.003), the fasting VPG level (χ2 = 186.155, P = 0.002) and BMI (χ2 = 100.910, P = 0.038). These factors were used as independent variables in the multivariate regression, as shown in Table 3. Multivariate regression showed that four of these variables affected the accuracy of non-invasive BG monitoring 2 h after a meal: fasting C-peptide (t = − 7.866, P = 0.000), HOMAIR (CP) (t = 7.745, P = 0.000), the VPG level 2 h after a meal (t = − 18.193· P = 0.000) and BMI (t = − 18.193, P = 0.000).
When the CEG was plotted 4 h after a meal, the measurements of 72 of 192 subjects with valid data fell in zone A; the remaining measurements fell outside of zone A. Based on the Chi-square test, seven variables were significantly different: fasting insulin (χ2 = 179.300, P = 0.004), fasting C-peptide (χ2 = 153.672, P = 0.001), HOMAβ (CP) (χ2 = 188.846, P = 0.002), HOMAIR (CP) (χ2 = 188.846, P = 0.002), the VPG level 4 h after a meal (χ2 = 177.825, P = 0.002), BMI (χ2 = 108.983, P = 0.010) and the disease course (χ2 = 40.923, P = 0.022). These factors were used as independent variables in the multivariate regression, as shown in Table 3. Multivariate regression showed that three of these variables significantly affected the accuracy of non-invasive BG monitoring 4 h after a meal: fasting C-peptide (t = − 8.085, P = 0.000), HOMAIR (CP) (t = 8.613, P = 0.000) and the VPG level 4 h after a meal (t = − 13.551, P = 0.000). Table 3 shows detailed data and Fig. 4 shows the multifactor analysis forest map.
Curves of HOMAIR–MARD and HOMAβ–MARD. BMI Basal metabolic index, CI Confidence interval, CP C-peptide, HOMAβ homeostatic model assessment model for β cell function, HOMAIR homeostatic model assessment model for insulin resistance, MARD mean absolute relative difference, ETD estimated treatment difference (Here means the difference of non-invasive glucose estimated accuracy)
When exogenous insulin is used, the HOMA index calculated from peripheral insulin cannot be used to describe insulin resistance. Therefore, in this study we use HOMA (CP) calculated by the FCP to decrease the impact of exogenous insulin. In addition, to make the conclusion more rigorous, HOMA analysis was also conducted specifically for non-insulin users. The results are shown in ESM Table S3, and the trend is the same as that shown in Table 3.
Discussion
The fingerstick capillary BG meter and CGM are used to determine the absolute value and trend of current BG levels, respectively. However, these methods cannot directly detect BG in blood or tissue fluid. In contrast, non-invasive BG measurement technologies, including the technology discussed in this study, provide a calculated BG value based on comprehensive processing of collected data collected, with the BG value inferred from the current actual BG measurement. When evaluating the accuracy of a non-invasive BG meter, the limitations and application of the technology adopted should be considered. For example, for people with suspicious impaired glucose regulation, NGM should be used to improve the sensitivity of monitoring of abnormal BG. However, when PWD use NGM to self-monitor BG, the focus should be on improving the accuracy in determining whether the actual BG level is higher than the target value, and further improving the sensitivity of identifying hypoglycaemia when possible. In this context, non-invasive BG monitoring methods should impact the clinical decision as little as possible.
The ISO15197:2013 standard is an accuracy evaluation standard applicable to BG monitoring systems used by the general public [24]. Such specific technical standards do not consider the actual effect of the current non-invasive BG monitoring technologies, so the provisions in the standard are more relevant to fingerstick BG meters. Because there are no recognised standards on the accuracy of non-invasive BG meters, in the present study we used some methods covered by the ISO15197:2013 to analyse our results.
CEG analysis is a comprehensive evaluation method that considers the accuracy and influence of variables on clinical decision-making. Each zone of the CEG represents a different degree of risk for the patient with diabetes according to the ISO 15197:2013 standard, with zone A representing “no effect on clinical action”; zone B, “altered clinical action—little or no effect on clinical outcome”; zone C, “altered clinical action—likely to affect clinical outcome”; zone D, “altered clinical action—could have significant medical risk”; and zone E, “altered clinical action—could have dangerous consequences”. When the results fall into zones A + B, the clinical outcome has little or no influence, but when the results fall into zone C, D or E, adverse clinical outcomes may thus increase [24]. In the present study, 93.9% of the data fell within zones A + B (with 54.3% in zone A), showing that overall there was no influence of the clinical outcome. The remaining 6.1% of the results fell within zones C + D, with no results in zone E, indicating that MHC-based non-invasive BG monitoring is a relative safe method to determine the proper clinical decision. The results of this investigation are consistent with those of previous single-centre studies. Our data are comparable to GlucoTrak measurements reported in Israel [27], although different methods were employed in that study.
In terms of the accuracy of a NGM, there are certain limitations when applying the ISO15197 standard, mainly represented by the allowance range of extreme BG values and the absolute value of the obtained data. Such limitations are obvious for hypoglycaemia and the MARD data in this study and the study on GlucoTrak [27]. For ethical considerations, hypoglycaemia data were collected passively in the NGM study without ‘creating’ greater hypoglycaemic episodes to subjects; it should be noted that extreme values can be ‘created’ by directly diluting BG samples for the fingerstick capillary BG meter and ,therefore, there was limited hypoglycaemia data in the present study. In the hypoglycaemic state, the body presents symptoms such as decreased extremity skin temperature, an increased blood flow rate and wet and cold skin due to increased sympathetic nerve excitability, all of which affect the parameters obtained with the MHC-based method. Hence, a non-invasive BG model for hypoglycaemia should be constructed independent of these symptoms. Moreover, the establishment and verification of a non-invasive BG model for hypoglycaemia faces great future challenges because some PWD suffer from hypoglycaemia-associated autonomic failure.
We analysed the factors affecting the accuracy of non-invasive BG monitoring at different time points. We found that gender, age and the disease course did not affect the accuracy of the non-invasive BG monitoring. Hence, we conclude that the MHC-based method is suitable for PWD regardless of their age, gender or disease course.
The results of our preliminary single-centre study showed the influence of fasting insulin and fasting C-peptide, while in the study itself, we introduced HOMA (CP) and other indexes for multivariate analysis. We found that the fasting C-peptide level was always an independent factor affecting the accuracy of non-invasive BG monitoring during fasting and at different times after a meal, indicating that the poorer endogenous insulin secretion, the lower the ability of the tissues to use glucose for metabolism. Consequently, we conclude that the C-peptide level significantly influences the accuracy of the MHC-based BG monitoring at all measurement times. HOMAIR is another independent factor that affects the BG measurements at different times after meals, but in the present study it did not influence fasting BG measurements. This finding suggests that the more severe the insulin resistance of the patients, the poorer the accuracy of the postprandial BG measurement. As insulin resistance increases, the efficiency of glucose metabolism in tissues gradually decreases, showing an independent influence on the accuracy of postprandial BG measurements. In addition, the fasting insulin level and BMI did not influence BG at the 4-h postprandial measurement compared with the independent influence of BMI on BG at the 2-h postprandial measurement, possibly due to its relevance to glucose absorption at the 4-h postprandial time point. However, the fasting C-peptide level and HOMAIR (CP) still significantly influenced BG at the 4-h postprandial time point, suggesting that these variables are better predictors of endogenous islet function and insulin resistance, respectively.
We built an individualised metabolic heat model for each subject by recording the personal FBG level on the screening day. Of note, multivariate analysis showed that the FBG level did not influence the accuracy of non-invasive BG monitoring. Considering that the VPG value at the corresponding time point was an independent factor that affected the BG measurements at the 2- and 4-h postprandial time points, it may be necessary to calibrate the 2-h postprandial BG to provide the best MHC-based non-invasive BG monitoring, for it might provide a better fit for determining the BG level than FPG. After a meal is consumed, insulin secretion surges and subsequently inhibits hepatic glucose production and promoting the transportation of glucose absorbed by the intestinal tract to peripheral tissues. In addition to the timing of postprandial BG calibration, the influence of meal type and other factors need to be studied, taking into consideration the postprandial timeliness of the aforementioned physiological changes.
To summarise, MHC-based non-invasive BG monitoring can meet the current need of daily self-monitoring by PWD. However, it requires further investigation and algorithm-based improvement in certain areas, including building a corresponding hypoglycaemia model, considering more patients with type 1 diabetes mellitus and optimising postprandial BG determination and the time interval between two continuous calibrations. Also, we believe that nowadays non-invasive BG monitoring should not be evaluated based on the same requirements for point-of-care glucometers or CGM.
Conclusions
The tested MHC-based mobile glucometer showed good clinical accuracy for BG measurement in Chinese PWD. It is a promising device for PWD in their daily BG monitoring. Further investigation of diabetes types, hormone levels, extreme samples and the non-equilibrium process of glucose metabolism may help to achieve greater accuracy of a MHC-based NGM and a broader useful range of BG measurements. This study provides a reference and help to develop in-depth investigations of non-invasive methods.
References
Wang L, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–23.
Xu Y, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–58.
Marrero DG, et al. Twenty-first century behavioral medicine: a context for empowering clinicians and patients with diabetes: a consensus report. Diabetes Care. 2013;36(2):463–70.
International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52.
American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S85–S99.
Zhou J, et al. The accuracy and efficacy of real-time continuous glucose monitoring sensor in Chinese diabetes patients: a multicenter study. Diabetes Technol Ther. 2012;14(8):710–8.
Bailey T. Clinical implications of accuracy measurements of continuous glucose sensors. Diabetes Technol Ther. 2017;19:S51–4.
Battelino T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603.
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Endocrinol Metab. 2021;37(4):311–98.
Vashist SK. Non-invasive glucose monitoring technology in diabetes management: a review. Anal Chim Acta. 2012;750:16–27.
Chen YH, et al. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci Adv. 2017;3(12):e1701629. https://doi.org/10.1126/sciadv.1701629.
Sieg A, Guy RH, Delgado-Charro MB. Electroosmosis in transdermal iontophoresis: implications for noninvasive and calibration-free glucose monitoring. Biophys J. 2004;87(5):3344–50.
Tamada JA, et al. Noninvasive glucose monitoring—comprehensive clinical results. JAMA. 1999;282(19):1839–44.
Ramasahayam S, et al. Noninvasive blood glucose sensing using near infra-red spectroscopy and artificial neural networks based on inverse delayed function model of neuron. J Med Syst. 2015;9(1):166. https://doi.org/10.1007/s10916-014-0166-2.
Goodarzi M, et al. Multivariate calibration of NIR spectroscopic sensors for continuous glucose monitoring. Trac-Trends Anal Chem. 2015;67:147–58.
Tavakoli M, et al. Corneal confocal microscopy. A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–7.
Hadar E, et al. Noninvasive, continuous, real-time glucose measurements compared to reference laboratory venous plasma glucose values. J Mater-Fetal Neonatal Med. 2019;32(20):3393–400.
Karpova EV, et al. Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal Chem. 2019;91(6):3778–83.
Caduff A, et al. Characteristics of a multisensor system for non invasive glucose monitoring with external validation and prospective evaluation. Biosens Bioelectron. 2011;26(9):3794–800.
Pleitez MA, et al. In vivo noninvasive monitoring of glucose concentration in human epidermis by mid-infrared pulsed photoacoustic spectroscopy. Anal Chem. 2013;85(2):1013–20.
Cho OK, et al. Noninvasive measurement of glucose by metabolic heat conformation method. Clin Chem. 2004;50(10):1894–8.
Feng R, et al. Noninvasive blood glucose monitor via multi-sensor fusion and its clinical evaluation. Sens Actuat B-Chem. 2021;332:129445.
Tang F, et al. Non-invasive glucose measurement by use of metabolic heat conformation method. Sensors. 2008;8(5):3335–44.
International Organization for Standardization (ISO). In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2013. https://www.iso.org/standard/54976.html.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95.
Keskin M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):E500–3.
Gal A, et al. Validity of GlucoTrack (R), a non-invasive glucose monitor, for a variety of people with diabetes. Diabet Med. 2013;30:69–69.
Acknowledgements
We thank all subjects who participated in this study and the physicians, nurses, clinical scientists and collaborators who enabled us to perform the study.
Funding
This study was financially supported by the National Natural Science Foundation of China (81873912) and Global Health Ark Medical Technology (Beijing) Co., Ltd. The latter company provided us with non-invasive glucometers but did not participate in the analysis process. We also thank the three research hospitals that provided us with trial support. No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ang Li, Chenyang Wu, Fei Tang and Zhanxiao Geng designed the trial. Ang Li, Xiaohui Guo and Junqing Zhang performed the clinical trial. Ang Li, Xiang Li, Yuanmeng Xuand Chenyang Wu wrote the paper. Ang Li, Xiang Li, Yuanmeng Xu, Chenyang Wu, Zhanxiao Geng, Xiaohao Wang and Fei Tang analysed the data. All authors interpreted the data and revised the paper. Ang Li and Xiang Li contributed equally
Disclosures
Ang Li, Xiang Li, Yuanmeng Xu, Chenyang Wu, Zhanxiao Geng, Junqing Zhang, Xiaohao Wang, Yuxiu Li, Hongmei Li, Xiaohui Guo, Fei Tang have nothing to disclose.
Compliance with Ethics Guidelines
The study was registered in the Chinese Clinical Trial Registry (Registration number ChiCTR1900020523). It was conducted simultaneously at the Peking University First Hospital, the China Meitan General Hospital and the Peking Union Medical College Hospital. The study protocol was approved by the Ethics Review Committee of Peking University First Hospital (ethics approval number: [2018] Device Registration No.10); the Ethics Review Committee of China Meitan General Hospital (ethics approval number: G18-11); and the Ethics Review Committee of Peking Union Medical College Hospital (ethics approval number: HS2018078). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all individual participants included in the study and their legal guardians.
Data Availability
Individual participant’s data will be shared in datasets in an anonymised format. Datasets of the VPG, non-invasive glucose level and the parameters measured by the NGM will be shared. The data will be available permanently after approval of the product and product use in China, the European Union and the United States. Data will be shared for researchers who submit a research proposal requesting access to the data. After approval of a proposal, the data will be sent by the corresponding author.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, A., Li, X., Xu, Y. et al. Evaluating the Clinical Accuracy of a Non-invasive Single-Fasting-Calibration Glucometer in Patients with Diabetes: A Multicentre Study. Diabetes Ther 14, 989–1004 (2023). https://doi.org/10.1007/s13300-023-01402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01402-8