Abstract
Introduction
Many individuals with type 2 diabetes (T2D) experience suboptimal glycemic control. Treatment intensification options include fixed-ratio combination products containing a basal insulin and a glucagon-like peptide-1 receptor agonist, such as iGlarLixi (insulin glargine 100 U/mL and lixisenatide). This study aimed to provide real-world evidence of the effect of iGlarLixi in Japanese clinical practice.
Methods
SPARTA Japan was a non-comparative, observational study conducted at 27 institutions in Japan. Anonymized individual-level data from adults with T2D receiving iGlarLixi in routine clinical practice were retrospectively collected. The primary study objective was to assess the impact of iGlarLixi on the change in glycated hemoglobin (HbA1c) at 6 months’ post-treatment initiation, with preplanned subanalyses to determine the influence of baseline characteristics. Secondary and exploratory endpoints included assessment of the proportion of individuals achieving HbA1c targets, change in body weight, and incidence and severity of hypoglycemia and gastrointestinal events.
Results
The full analysis set included 432 individuals, with data available at 6 months for 426. Of the 432 individuals, the mean (SD) age at baseline was 61.6 (12.8) years and the majority had a T2D duration of ≥ 10 years [mean (SD) 13.3 (10.4) years]. At 6 months, HbA1c had significantly decreased versus baseline ( –0.85%; P < 0.0001), with a greater decrease in those aged < 65 years, with a shorter duration of T2D and higher baseline HbA1c. A significant increase in the proportion of participants achieving age-specific HbA1c versus baseline was observed. Mean body weight decreased by 0.5 kg (P = 0.0034 versus baseline). There were few hypoglycemia and gastrointestinal events (in individuals with HbA1c data); no severe hypoglycemic events were reported.
Conclusions
The results of this real-world study indicate that iGlarLixi may improve glycemic control without serious adverse events in Japanese individuals with T2D who have suboptimal glycemic control on current treatment regimens and switch to iGlarLixi.
Trial registration
UMIN-CTR Trials Registry, UMIN000044126; registered 10 May 2021.
Plain Language Summary
The first medicines for treating diabetes that many individuals with type 2 diabetes receive are administered orally; however, for most individuals, these oral drugs are not enough to achieve blood glucose targets as their disease progresses. Treatment intensification options include adding an injectable therapy, such as a glucagon-like peptide-1 receptor agonist or basal insulin, or the combination of both, the use of which has been studied extensively and has been shown to be a simple and well-tolerated option. Here, we report the findings of a study that retrospectively evaluated the outcomes of 432 Japanese individuals who took iGlarLixi, which consists of the glucagon-like peptide-1 receptor agonist lixisenatide and basal insulin glargine 100 U/mL as a fixed-ratio combination (i.e., combined as a single subcutaneous injection), over 6 months of treatment. We found that iGlarLixi improved blood glucose levels in these individuals, and was associated with few hypoglycemia or gastrointestinal adverse events. These results suggest that iGlarLixi may offer an effective option for improving glycemic control in Japanese individuals with type 2 diabetes who require treatment intensification because their blood glucose goals have not been achieved with oral drugs alone or co-administered with a glucagon-like peptide-1 receptor agonist or basal insulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Combination products containing a fixed ratio of basal insulin to glucagon-like peptide-1 receptor agonist (GLP-1 RA) have been developed to improve glycemic control, with good tolerability and a reduced injection burden in individuals with type 2 diabetes (T2D) requiring treatment intensification. |
iGlarLixi, containing insulin glargine 100 U/mL and a GLP-1 RA, lixisenatide, has been approved for the treatment of T2D in a number of countries, including Japan. |
Real-world data of the effectiveness and safety of iGlarLixi in Japanese clinical practice are lacking. |
What was learned from the study? |
The results of this non-comparative, observational study suggest that the use of iGlarLixi in a real-world setting was associated with improved glycemic control, as evidenced by a significant reduction in glycated hemoglobin (HbA1c) and an increase in the proportion of study participants achieving target HbA1c. |
iGlarLixi appeared to be well tolerated; there were few hypoglycemia and gastrointestinal events, and those that did occur were mostly mild to moderate in severity and rarely required specific treatment. |
Introduction
Diabetes is a globally important disease. In 2021, there were estimated to be 537 million adults living with diabetes worldwide, with this number expected to reach 783 million by 2045 [1]. In 2019, it was estimated that 7.39 million individuals in Japan (5.6% of the population) were diagnosed with type 2 diabetes (T2D) [2], where a Western lifestyle and an aging population are likely contributing to an increase in the incidence of the disease.
Basal insulin therapy or glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are options for treatment intensification in individuals whose diabetes is suboptimally controlled with oral antidiabetic drugs (OADs) alone [3,4,5]. However, insulin therapy is associated with a risk of hypoglycemia [6] and body weight gain [7], and gastrointestinal side effects are of concern with GLP-1 RAs [8]. Further, neither insulins nor GLP-1 RAs alone nor as dual therapy with OADs achieve glycated hemoglobin (HbA1c) targets (frequently considered as < 7%) in all individuals [9]. In an attempt to improve outcomes, insulin therapy and GLP-1 RAs are often co-administered; however, such therapy requires two separate injections, creating a significant treatment burden on individuals. In part because of the above reasons, in current clinical practice, treatment intensification to better manage glycemic levels is often delayed and suboptimal in those individuals who may benefit from it [10, 11].
Fixed-ratio combinations (FRC) of basal insulin and a GLP-1 RA have been developed with the aim of providing effective glycemic control, better tolerability than either agent alone, and a reduced injection burden to enhance treatment adherence and persistence. iGlarLixi is a once-daily titratable FRC of insulin glargine 100 U/mL, a long-acting basal insulin analog, and a GLP-1 RA, lixisenatide. iGlarLixi is available in the USA in a dose ratio of 3 U/mL:1 μg for the two components, respectively [12], and in Europe in ratios of 2 U/mL:1 μg and 3 U/mL:1 μg, respectively, for the treatment of adults with T2D that is inadequately controlled by other treatments [13]. In 2020, iGlarLixi (one dose step = glargine 100 U/mL:1 μg lixisenatide) was approved and launched in Japan [14] for the treatment of individuals with T2D where insulin therapy is indicated. The approval of iGlarLixi in Japan was based on data from three randomized, controlled clinical trials (LixiLan JP-O1 [15], LixiLan JP-O2 [16], and LixiLan JP-L [17]) that involved Japanese individuals with T2D who had had a suboptimal treatment response to OADs [15, 16] or to OADs plus basal insulin [17]. While these trials demonstrated the efficacy and safety of iGlarLixi, real-world data of its effectiveness and safety in Japanese clinical practice are currently lacking. Such data, in addition to robust clinical trial data, would support physicians in making clinical decisions on individualized treatment for Japanese individuals with T2D.
The “SPARTA Japan” study aimed to retrospectively evaluate the effect of iGlarLixi on HbA1c in individuals with T2D in routine clinical practice in Japan. This observational study also aimed to assess the impact of iGlarLixi on the incidence of hypoglycemia, a common adverse event (AE) with insulin therapy, and gastrointestinal events, which are common with GLP-1 RAs. The effect of iGlarLixi on body weight was also assessed, since body weight gain often occurs during insulin therapy [7].
Methods
Study Design
SPARTA Japan was a retrospective, non-comparative, observational study conducted at 27 institutions that were geographically spread across Japan and represented different regions. Clinicians at the participating institutions were among the regular prescribers of iGlarLixi. Supplementary Fig. S1 in the electronic supplementary material provides an outline of the study design.
Anonymized individual-level data were retrospectively collected from electronic medical records and paper charts, with the use of an electronic case report form.
The baseline period was defined as the 6 months prior to initiation of iGlarLixi treatment, and the observation period as the 6 months after initiation of treatment. Baseline data were defined as the data closest to the start date of iGlarLixi within 6 months before the initiation of treatment, with the exception of HbA1c, which was defined as the closest data to the start of iGlarLixi within 3 months prior to treatment initiation.
HbA1c and body weight were assessed at 3 and 6 months after initiation of iGlarLixi. Because this was an analysis of data collected during routine clinical practice, assessments were not necessarily conducted at exactly 3 and 6 months after initiation of iGlarLixi. Therefore, data collected from 45 to 135 days and 136–225 days after the start of treatment could be included in the 3- and 6-month data analysis, respectively.
The incidence and severity of hypoglycemia and gastrointestinal events were assessed over the course of the 6-month observation period, as were iGlarLixi dose changes. The evaluation period for hypoglycemia and gastrointestinal events was from day 1 of iGlarLixi administration to day 3 of HbA1c measurement at 3 and 6 months after the start of administration, with day 1 being the day following the start of iGlarLixi treatment.
Study Population
The individuals included in the study were treated in routine clinical practice according to the iGlarLixi prescribing information and reimbursement criteria at the treating physicians’ discretion. The inclusion criteria were adults who had (i) a diagnosis of T2D; (ii) initiated treatment with iGlarLixi ≥ 6 months before data collection, regardless of treatment continuation or discontinuation; (iii) at least one HbA1c recording in the 3 months prior to initiation of treatment with iGlarLixi; (iv) body weight recorded in the 6 months prior to treatment initiation; (v) at least one HbA1c recording in the 6 months after treatment initiation; and (vi) provided informed consent. The exclusion criteria were: (i) aged < 18 years at initiation of iGlarLixi; (ii) type 1 diabetes; (iii) pregnancy during the observation period; (iv) previous participation in any iGlarLixi trials or enrollment in any clinical trial related to diabetes during the observation period; (v) off-label use of iGlarLixi (including concomitant use of basal insulin); or (vi) use of insulin degludec/liraglutide (IDegLira) within 3 months prior to initiation of iGlarLixi.
Study Objectives and Endpoints
The primary study objective was to evaluate the impact of iGlarLixi on HbA1c, measured from baseline to 6 months after treatment initiation, in routine clinical practice in Japan. Subgroup analyses were performed to assess the influence of baseline characteristics [age, duration of T2D, body mass index (BMI), and HbA1c] on the change in HbA1c, body weight, iGlarLixi dose, and the incidence of hypoglycemia and gastrointestinal events at 6 months.
The secondary study objectives were to assess: the change in HbA1c from baseline to 3 months after treatment initiation; the proportion of individuals achieving a target HbA1c of < 7.0% (< 53 mmol/mol); and the proportion of individuals achieving personalized HbA1c targets by age category [< 65 years = < 7.0%; 65 to < 75 years = < 7.5% (< 58 mmol/mol); ≥ 75 years = < 8.0% (< 64 mmol/mol)] according to the Japanese Diabetes Society [5]. These age-specific targets were based on the avoidance of diabetic complications, and were set to take into account the individuals’ age, duration of diabetes, risk for hypoglycemia (which increases with age), available support, cognitive function, basic/instrumental activities of daily living, and comorbidities/functional impairments [5].
Exploratory analysis was performed to determine (i) the change in body weight from baseline to 3 and 6 months after initiating treatment; (ii) the cumulative incidence and severity of hypoglycemia at 3 and 6 months; (iii) the cumulative incidence and severity of gastrointestinal events at 3 and 6 months; (iv) the change in dose of iGlarLixi; (v) the reasons for initiation and discontinuation of iGlarLixi; and (vi) the factors affecting HbA1c at 6 months’ post-treatment initiation.
Self-reported hypoglycemic events were classified as severe (i.e., when neurological impairment was severe enough to prevent self-treatment and, thus, thought to place the participant at risk for injury to themselves or others); symptomatic (i.e., accompanied by typical symptoms of hypoglycemia); asymptomatic (i.e., not accompanied by typical symptoms of hypoglycemia); and possibly symptomatic (i.e., accompanied by typical symptoms of hypoglycemia but with no measured plasma glucose levels at the onset of hypoglycemic symptoms). These hypoglycemic events were further classified based on a measured plasma glucose concentration of ≤ 70 mg/dL (3.9 mmol/L) or < 54 mg/dL (3.0 mmol/L) when data were available.
Compliance with Ethics Guidelines
Individuals provided written consent for their data to be used in the study. Ethics committee approval for the study was applied for and granted prior to study initiation by a central ethics committee (Sone Clinic in Tokyo, Japan; approval granted 24 February 2021) for some institutions and by their own institutional ethics committee in other institutions (Supplementary Table S1).
Statistical Analysis
The required sample size was estimated to be 388 individuals. This calculation was based on the assumption of a mean HbA1c reduction of 0.60% (as per the findings of a real-world study of IDegLira [18] and the GLP-ONE Kobe study of insulin glargine plus lixisenatide or vildagliptin [19]) and a standard deviation (SD) of 1.5% from baseline to 6 months after iGlarLixi initiation in routine clinical practice, with a precision of 0.15% and a confidence interval (CI) of 95%. Additionally, assuming the total proportion of individuals who discontinued treatment or had missing data to be approximately 15%, it was estimated that 460 individuals would need to be enrolled to ensure a final sample size of 388.
The individuals who met all inclusion criteria and did not meet any exclusion criteria were included in the full analysis set (FAS). Individuals with data at the time of evaluation were analyzed, regardless of whether treatment was interrupted or discontinued. Data from certain time points were not included in the analysis from individuals who took > 20 dose steps/day of iGlarLixi at that specific time point during the observation period.
Summary statistics of baseline characteristics were calculated, such as mean with SD and/or 95% CIs, minimum and maximum, median, and interquartile range (IQR). In hypothesis testing, a P-value < 0.05 was considered statistically significant. Changes in HbA1c and body weight were tested with a paired t-test.
Baseline characteristics possibly impacting changes in HbA1c were explored in two ways. Firstly, individuals were stratified by baseline age (two cutoffs: < 65 and ≥ 65 years; < 75 and ≥ 75 years), duration of T2D (< 10 and ≥ 10 years), BMI (< 25, ≥ 25 to < 30, and ≥ 30 kg/m2), and HbA1c [two cutoffs: < 8.0% and ≥ 8.0%; < 9.0% and ≥ 9.0% (< 75 and ≥ 75 mmol/mol)]. Secondly, multivariate regression analysis was performed to examine potential factors affecting HbA1c, with the change in HbA1c from baseline to 6 months as the dependent variable and baseline HbA1c, duration of T2D, BMI, age, sex, and change in body weight from baseline as independent variables. Subsequently, differences in HbA1c change from baseline to 6 months between or among these specified subgroups were evaluated using regression models with adjustment for the factors that were found statistically significant.
Multivariate logistic regression analysis was also performed to examine the same potential factors affecting the achievement of a targeted HbA1c, with the proportion of participants reaching an HbA1c < 7.0% or an individualized HbA1c target at 6 months after the initiation of iGlarLixi as the dependent variable.
The impact of baseline characteristics on changes in body weight and iGlarLixi dose, and the incidence of hypoglycemia and gastrointestinal events at 6 months was assessed as per the stratification outlined above for changes in HbA1c (i.e., by baseline age, duration of T2D, BMI, and HbA1c). A paired t-test was used to evaluate the impact of baseline characteristics on body weight and iGlarLixi dose, using data from study participants who had data at both baseline and the 6-month time point. Analyses were descriptive only for the incidence of hypoglycemia and gastrointestinal events.
No adjustment for multiplicity was made in the current study. Missing values were not imputed. Statistical analysis was performed using SAS Version 9.4 (SAS Institute; Cary, NC, USA).
Results
Study Cohort
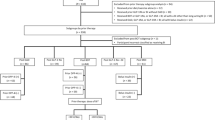
Data collection, extraction, and analysis were performed between June and September 2021. In total, 469 individuals with T2D were eligible for inclusion; after medical records were assessed against the inclusion/exclusion criteria, 432 patients were included in the FAS (Fig. 1). Among the 37 individuals who were excluded from the FAS, the most common reasons for exclusion were as follows: missing data during the baseline or observational periods (n = 10), concomitant use of basal insulin (n = 13), and concomitant use of dipeptidyl peptidase-4 inhibitors (DPP-4i) (n = 7).
Flow of individuals through the observational, non-comparative SPARTA Japan study. BI basal insulin, BMI body mass index, DPP-4i dipeptidyl peptidase-4 inhibitors, FAS full analysis set, HbA1c glycated hemoglobin, iGlarLixi insulin glargine/lixisenatide, IDegLira insulin degludec/liraglutide, mo months, SPARTA-J SPARTA Japan, yrs years
Of the 432 individuals included in the FAS, 427 (98.8%) had data available for baseline and at 3 months, and 426 (98.6%) had data available at 6 months’ post-treatment initiation.
Demographics
The mean (SD) age at baseline was 61.6 (12.8) years, with over half (56.9%) of the study cohort in the < 65-years age category (Table 1). The mean (SD) body weight was 71.8 (15.9) kg and BMI was 26.8 (4.6) kg/m2 at baseline. The mean duration of T2D in the study cohort was approximately 13 years, with the majority (55.8%) having had the disease for ≥ 10 years. Diabetes-related complications were present in over half of participants (54.9%). The mean (SD) HbA1c at baseline was 8.96 (1.64) %, and almost half the participants (41.9%) had a baseline HbA1c ≥ 9.0%.
At baseline, 333 participants (77.1%) were taking one or more injectable agents to manage their diabetes (Table 1). The most common formulation was long-acting basal insulin, which was being used by 226 participants (52.3%). GLP-1 RA, premixed/combination insulin, and rapid-acting insulin were being taken by 143 (33.1%), 60 (13.9%), and 53 (12.3%) study participants, respectively. The mean (SD) baseline daily dose of basal insulin was 11.9 (5.7) U.
In total, 366 participants (84.7%) were taking concomitant OADs to manage their T2D at baseline (Table 2). Most participants were taking one [n = 102 (23.6%)] or two [n = 169 (39.1%)] OADs. The most common classes of OADs taken were biguanides and sodium-glucose transport protein 2 (SGLT2) inhibitors.
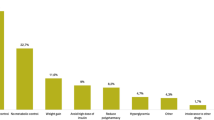
The most common reasons for initiating iGlarLixi were suboptimal control of HbA1c (88.2% of participants) or postprandial glucose levels with previous treatment (38.7% of participants; Supplementary Table S2). Body weight gain on previous treatment (19.2%) and a wish to reduce the number of injections (8.8%) were also provided as reasons for initiating iGlarLixi. The median (IQR) duration of iGlarLixi treatment was 143.5 (101.3) days.
Primary Endpoint
Data for HbA1c at 6-months post-initiation of iGlarLixi were available for 418 study participants. At this time-point, the mean (95% CI) HbA1c was 8.12% (7.99%, 8.26%), a decrease of –0.85% (–1.01%, –0.68%) compared with baseline (P < 0.0001; Fig. 2).
Baseline age appeared to have a variable relationship with change in HbA1c, with a significant difference in those < 65 years versus those ≥ 65 years, but a similar relationship was absent in the < 75 years versus ≥ 75 years’ comparison (Fig. 3; Supplementary Table S3). The duration of T2D also appeared to be inversely related to change in HbA1c, with a greater decrease seen in participants with a shorter duration (< 10 years) of disease. In contrast, baseline BMI had no apparent association with change in HbA1c. A greater decrease in HbA1c was observed in those participants with a higher baseline HbA1c. These results were confirmed in the multivariate regression analysis, where age, duration of T2D, and baseline HbA1c were shown to impact HbA1c change at 6 months (Table 3).
Subgroup analyses for unadjusted change in mean glycated hemoglobin at 6 months’ post-initiation of insulin glargine/lixisenatide by baseline characteristics. Error bars represent 95% confidence intervals. Adjusted change is shown in Supplementary Table S3. BMI body mass index, HbA1c glycated hemoglobin, T2D type 2 diabetes. *P < 0.01 versus baseline; **P < 0.001 versus baseline; ***P < 0.0001 versus baseline
Secondary Endpoints
Data for HbA1c at 3 months’ post-initiation of iGlarLixi were available for 415 study participants. At this time point, the mean HbA1c had decreased significantly from baseline [mean (95% CI) change –0.64% ( –0.80%, –0.48%); P < 0.0001; Fig. 2). Three months after initiating iGlarLixi, 14.4% of the study cohort (n = 62) had reached an HbA1c < 7.0% (compared with 5.6% at baseline). At the 6-month follow-up point, this proportion had increased further to 18.5% (Supplementary Fig. S2).
In each age group, there was a significant increase in the proportion of participants who reached the age-specific HbA1c target at each study time point versus baseline (Fig. 4). For example, the percentage of participants aged < 65 years (n = 246) who were at their age-specific HbA1c (< 7.0%) increased from 4.9% at baseline to 19.1% at 6 months. Similar changes were seen in values for those aged ≥ 65 to < 75 years (n = 121) and ≥ 75 years (n = 65), although the increase from baseline was not as marked in these groups as in those study participants < 65 years.
Regarding the factors associated with the achievement of an HbA1c of < 7.0% at 6 months of treatment with iGlarLixi, the multivariate analysis indicated that duration of T2D and change in body weight from baseline were the only factors linked to this (data not shown).
Other Exploratory Endpoints
Mean (95% CI) body weight decreased from 71.8 (70.3, 73.3) kg at baseline to 71.3 (69.8, 72.8) kg at 3 months (P = 0.0001). This 0.5 kg body weight loss was maintained at 6 months [mean (95% CI) 71.3 (69.8, 72.9) kg; P = 0.0034 versus baseline).
At the 3-month time point, 32 study participants (7.7%) had experienced 94 hypoglycemic events, giving an overall incidence of 0.9 events/person-year of exposure to iGlarLixi (Table 4). This incidence rate had dropped slightly at the 6-month time point, when hypoglycemia had been reported in an additional 60 participants (14.4%), with an overall incidence of 0.8 events/person-year. No severe hypoglycemic events were reported, and no participants visited the emergency department or were hospitalized because of hypoglycemia.
At the 3- and 6-month time-points, 62 (14.9%) and 68 (16.3%) study participants, respectively, had experienced gastrointestinal events (Table 5). The overall incidence rate was 0.9 events/person-year at 3 months and 0.5 events/person-year at 6 months. The most common events were nausea, constipation, diarrhea, and abdominal discomfort, but none had an incidence rate of > 0.3 events/person-year.
At 6 months, 56 study participants (13.0%) had discontinued iGlarLixi. The most common reasons given for discontinuation were insufficient HbA1c control and gastrointestinal events (Supplementary Table S4). There were no deaths recorded during the study, and no cases of treatment-related pancreatitis or malignancy. No participants discontinued treatment because of body weight gain, and none cited cost as a factor influencing the decision to discontinue treatment.
Mean (95% CI) daily iGlarLixi dose increased from 8.6 (8.2, 9.0) dose steps at the initial dose to 12.2 (11.7, 12.7) dose steps at the last dose (P < 0.0001; n = 432). Furthermore, the mean (95% CI) daily basal insulin dose in participants who had previously been on basal insulin therapy increased from 11.9 (11.2, 12.7) U at baseline to 13.1 (12.4, 13.8) U at the last dose (P = 0.0003; n = 224).
The subgroup analyses for the impact of baseline characteristics on change in body weight and iGlarLixi dose, and the incidence of hypoglycemia and gastrointestinal events are presented in Supplementary Tables S5–S7. Study participants who were of a younger age and a higher baseline BMI had a more marked decrease in body weight at 6 months. Baseline HbA1c had a variable effect on body weight (Supplementary Table S5). There was a smaller change in iGlarLixi dose from baseline to last dose in study participants who were older and who had a shorter duration of T2D (Supplementary Table S6). The incidence of all and symptomatic hypoglycemic events tended to be higher in study participants who were older at baseline, and who had a longer duration of T2D and a lower baseline BMI; baseline HbA1c did not have any discernible effect. In general, a similar pattern was seen with the incidence of gastrointestinal events (Supplementary Table S7).
Discussion
The results of this study suggest that, when used in a real-world setting, iGlarLixi may improve glycemic control without serious adverse events in Japanese individuals with T2D that is suboptimally controlled with their previous diabetes treatments. Treatment with iGlarLixi for 6 months was associated with a significant reduction in HbA1c versus baseline, and a significant increase in the proportion of study participants achieving an HbA1c < 7.0% and achieving age-specific HbA1c targets versus baseline. The study results also suggest that iGlarLixi was well tolerated; there were few hypoglycemia and gastrointestinal events, and those that did occur were mostly mild to moderate in severity, and rarely required specific treatment. Further, there were no reported cases of severe hypoglycemia. Few participants discontinued iGlarLixi treatment and, in those who did, AEs were rarely the reason (2.5% discontinued due to gastrointestinal events). Body weight decreased by an average of 0.5 kg during the 6-month observation period.
The findings of this study, including the rates of hypoglycemia and gastrointestinal events, support those obtained from the three 26-week, open-label clinical trials of iGlarLixi in Japanese individuals with T2D (LixiLan JP-O1 [15], LixiLan JP-O2 [16], and LixiLan JP-L [17]). The magnitude of HbA1c improvements from baseline and the proportion of individuals who achieved a target HbA1c of < 7.0% was greater in these clinical trials ( –1.27 to –1.58, and 51.8–71.5%, respectively) than in our real-world study ( –0.85% and 18.5% at 6 months, respectively). This is likely to be the result of a number of factors, including a less heterogeneous study population and better treatment adherence in clinical trials, and clinical inertia (i.e., a failure of clinicians to intensify treatment in a timely manner) and the less stringent titration used in clinical practice compared with the trials.
Information on the possible influence of background characteristics may be useful to guide treatment decisions in routine clinical practice, in particular, to personalize treatment strategies in individuals who have T2D with suboptimally controlled blood glucose levels. Our study explored the impact of baseline age, disease duration, BMI, and HbA1c on the change in HbA1c at 6 months. Shorter disease duration and higher baseline HbA1c were associated with a greater reduction in HbA1c. In contrast, age showed a variable association in our study cohort with regard to the change in HbA1c, while baseline BMI did not appear to influence HbA1c. When subdivided into categories of < 65 years or ≥ 65 years, age was significantly inversely associated with HbA1c change during iGlarLixi, but the same trend was not observed when the categories of < 75 years versus ≥ 75 years were compared. This observation may, in part, reflect differential HbA1c targets according to age in the Japanese guidelines [5], where more aggressive treatment of younger individuals (< 65 years) to a lower HbA1c target may be needed compared with a more cautious approach to older individuals (≥ 75 years), who are at high risk of hypoglycemia.
Apart from that reported herein, there is currently only limited real-world evidence of the effectiveness and safety of iGlarLixi, particularly in Japanese individuals. A single-center study assessed the effect of 24 weeks of treatment with iGlarLixi in Japanese individuals (n = 40) with a suboptimal response to OADs [20]. Mean (SD) HbA1c decreased from 9.4 (1.4) % at baseline to 6.4 (0.9) % at the end of the observation period (P < 0.001). The incidence of hypoglycemia (number of times hypoglycemic symptoms occurred with a confirmed blood glucose < 70 mg/dL) decreased from a mean (SD) of 5.0 (2.8) times during weeks 5–8 to 3.6 (2.1) times during weeks 21–24, there were no serious AEs (including gastrointestinal events), and no participant discontinued treatment during the study.
Some data are available from real-world studies conducted in other countries. An analysis of the effects of concomitant iGlarLixi and SGLT2 inhibitor therapy included data from a retrospective, observational cohort study using information captured by the US Optum-Humedica database [21]. One arm of this study consisted of adults who used iGlarLixi monotherapy. In this group (n = 1054), mean HbA1c decreased by 1.05% versus baseline after 6 months of treatment. The incidence of hypoglycemia was 0.24 events/person-year [21]. These changes in HbA1c were similar to those in our study, but the incidence of hypoglycemia was lower. The variation in results may be due to the differences in sample size, study design, and included populations (e.g., most participants in our study were not on iGlarLixi monotherapy). Despite the difference in the formulation of iGlarLixi used in the USA versus Japan, the magnitude of HbA1c reduction over 6 months was similar in the US study [21] to that in our study. This may be partly explained by differences in the pathophysiology of T2D in Japanese individuals versus non-Japanese individuals. Postprandial glucose control is of greater significance in Japanese individuals because of pronounced β-cell dysfunction and reduced insulin secretion compared with Caucasians [22]. The 1:1 Japanese iGlarLixi formulation addresses the need for both postprandial glucose and fasting plasma glucose control, yielding a clinically relevant reduction in HbA1c.
Our study has a number of strengths. It provides real-world data on the use of iGlarLixi to treat T2D, reflecting actual clinical practice and the experience of individuals with T2D in Japan, in contrast to the data obtain from tightly controlled clinical trials. Further, it had a moderately large sample size and included individuals with various treatment backgrounds and comorbidities representing a broader Japanese T2D population. However, the study does have a number of limitations that need to be considered when interpreting the results. First, being a retrospective observational study, participants were not randomized to treatment with iGlarLixi, some potential confounders (such as comorbidities) were not assessed or accounted for, and there may have been physician selection and enrollment bias. Further, the study did not include a comparator and so the relative effectiveness of iGlarLixi compared with other treatment modalities for T2D cannot be determined from our study results. Second, the study only assessed the effects of iGlarLixi treatment for 6 months, which is a relatively short treatment period for T2D, a chronic disorder. Third, some of the study subgroups were of a small size, such as those of study participants ≥ 75 years (n = 65) and with a BMI ≥ 30 kg/m2 (n = 95). Fourth, treatment discontinuations due to gastrointestinal events may have been underestimated, as the first assessment of such effects was only recorded for individuals if they had data at 3 months’ post-initiation of iGlarLixi. Gastrointestinal events are known to occur early in the course of iGlarLixi treatment and, therefore, study participants particularly affected by such AEs may have discontinued the drug before the 3-month time point. Fifth, the study did not incorporate an inclusion criterion for participants to have been on stable antidiabetic medication in the 6 months prior to study enrollment. Hence, it is possible that the observed effects were related to changes in (intensification of) other antidiabetic medications rather than the initiation of iGlarLixi. However, it should be noted that the proportion of individuals who had changes in the dose of their OADs or bolus insulin after initiation of iGlarLixi was relatively small (data not shown). Finally, the findings from this Japanese sample may not be applicable to individuals with T2D in other countries, particularly given that the ratio of the two components of iGlarLixi used in Japan differs to that used in the USA and Europe.
Conclusions
The results of this real-world study generally support those reported from randomized controlled trials of iGlarLixi in Japan. The current study’s findings indicate that iGlarLixi improved glycemic control without serious adverse events in Japanese individuals with T2D who have suboptimal glycemic control on current treatment regimens in clinical practice. A significant HbA1c reduction from baseline was observed in the FAS.
References
International Diabetes Federation. Diabetes facts and fifures. 2021. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed 24 Feb 2022.
Kohsaka S, Morita N, Okami S, Kidani Y, Yajima T. Current trends in diabetes mellitus database research in Japan. Diabetes Obes Metab. 2021;23(Suppl 2):3–18.
American Diabetes Association. Standards of medical care in diabetes - 2022. Diabetes Care. 2022;45:S1–2.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461–98.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11:1020–76.
Heller SR, Peyrot M, Oates SK, Taylor AD. Hypoglycemia in patient with type 2 diabetes treated with insulin: it can happen. BMJ Open Diabetes Res Care. 2020;8(1): e001194.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812.
Anderson SL, Trujillo JM. Lixisenatide in type 2 diabetes: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2016;7:4–17.
Baxter M, Morimoto Y, Tamiwa M, et al. A real-world observational study evaluating the probability of glycemic control with basal insulin or glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes. Diabetes Ther. 2020;11:1481–96.
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–7.
Maegawa H, Ishigaki Y, Langer J, Saotome-Nakamura A, Andersen M. Japan Diabetes Clinical Data Management Study G. Clinical inertia in patients with type 2 diabetes treated with oral antidiabetic drugs: results from a Japanese cohort study (JDDM53). J Diabetes Investig. 2021;12:374–81.
US Food and Drug Administration. Soliqua prescribing information. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208673s000lbl.pdf. Accessed 24/02/2022.
European Medicine Agency. Suliqua (insulin glargine / lixisenatide). 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/suliqua. Accessed 24/02/2022.
Pharmaceuticals and Medical Devices Agency Japan. New Drugs Approved in FY 2019. 2020. https://www.pmda.go.jp/files/000235289.pdf. Accessed 24 Feb 2022.
Watada H, Takami A, Spranger R, Amano A, Hashimoto Y, Niemoeller E. Efficacy and safety of 1:1 fixed-ratio combination of insulin glargine and lixisenatide versus lixisenatide in Japanese patients with type 2 diabetes inadequately controlled on oral antidiabetic drugs: the LixiLan JP-O1 randomized clinical trial. Diabetes Care. 2020;43:1249–57.
Terauchi Y, Nakama T, Spranger R, Amano A, Inoue T, Niemoeller E. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi 1:1) in Japanese patients with type 2 diabetes mellitus inadequately controlled on oral antidiabetic drugs: a randomized, 26-week, open-label, multicentre study: the LixiLan JP-O2 randomized clinical trial. Diabetes Obes Metab. 2020;22(Suppl 4):14–23.
Kaneto H, Takami A, Spranger R, Amano A, Watanabe D, Niemoeller E. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi) in Japanese patients with type 2 diabetes mellitus inadequately controlled on basal insulin and oral antidiabetic drugs: the LixiLan JP-L randomized clinical trial. Diabetes Obes Metab. 2020;22(Suppl 4):3–13.
Melzer-Cohen C, Chodick G, Naftelberg S, Shehadeh N, Karasik A. Metabolic control and adherence to therapy in type 2 diabetes mellitus patients using IDegLira in a real-world setting. Diabetes Ther. 2020;11:185–96.
Otowa-Suematsu N, Sakaguchi K, Nakamura T, et al. Comprehensive evaluation of combination therapy with basal insulin and either Lixisenatide or Vildagliptin in Japanese patients with Type 2 Diabetes: a randomized, open-label, parallel-group. Multicenter Study Diabetes Ther. 2018;9:2067–79.
Daikuhara H. Usefulness of Soliqua® injection for the introduction of new injectable drugs for diabetes mellitus: results of a 24-week trial in 40 patients with type 2 diabetes [in Japanese]. Med Consult New Remedies. 2021;58:605–15.
Guja C, Giorgino F, Blonde L, et al. Concomitant iGlarLixi and sodium-glucose co-transporter-2 inhibitor therapy in adults with type 2 diabetes: LixiLan-G trial and real-world evidence results. Diabetes Ther. 2022;13:205–15.
Yabe D, Seino Y, Fukushima M, Seino S. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:602.
Acknowledgements
We would like to thank the following institutions for their involvement in this study: Hamamatsu Medical Center, National Hospital Organization Hyogo-Chuo National Hospital, Medical Corporation Jinnouchi-kai Jinnouchi Hospital, Suzuki Internal Medicine and Diabetes Clinic, Nagoya City University Hospital, Medical Corporation Ayame Medical Clinic, Medical Corporation Kouseikai Iwamoto Internal Medicine Clinic, Primula Clinic, Medical Corporation Towa-kai Kikuchi Medical Clinic, Medical Corporation Hayaishi-kai Hayaishi Hospital, Japan Organization of Occupational Health and Safety, Kansai Rosai Hospital, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Medical Corporation Omotokai Ohama Dai-ichi Hospital, Medical Corporation Touyukai Kurihara Internal Medicine, Medical Corporation Muraki Internal Medicine and Gastroenterology Clinic, Internal Medicine Kokubo Clinic, General Incorporated Association of Hygiene and Culture Johsai Hospital, Osaka City General Hospital, Medical Corporation Morinoki-kai Morinoki Clinic, Seino Internal Medicine Clinic, Nishikawa Clinic, Social Welfare Organization Saiseikai Imperial Gift Foundation, Shizuoka Saiseikai General Hospital, Jichi Medical University Saitama Medical Center, Social Medical Corporation Shiseikai Inuyama Chuo General Hospital, Miyachi Clinic, Kasugai Municipal Hospital, and the Japan Organization of Occupational Health and Safety, Ehime Rosai Hospital
Funding
Sponsorship for this study, medical writing assistance, and the journal’s Rapid Service Fee were funded by Sanofi K.K.
Medical Writing, Editorial and Other Assistance
We would like to thank Kate Palmer of inScience Communications, Springer Healthcare, who wrote the outline and first draft of the manuscript, and assisted with post-submission revisions. Support for this medical writing assistance was funded by Sanofi K.K.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study concept and design: Munehide Matsuhisa, Hideaki Miyoshi, Daisuke Yabe, Yoko Takahashi, Yukiko Morimoto, and Yasuo Terauchi; Data acquisition: Yoko Takahashi; Data analysis and interpretation: Munehide Matsuhisa, Hideaki Miyoshi, Daisuke Yabe, Yoko Takahashi, Yukiko Morimoto, and Yasuo Terauchi; Writing-review and editing: Munehide Matsuhisa, Hideaki Miyoshi, Daisuke Yabe, Yoko Takahashi, Yukiko Morimoto, and Yasuo Terauchi.
Disclosures
Munehide Matsuhisa has received honoraria from Sanofi K.K., Takeda Pharmaceutical, Eli Lilly Japan, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma, Novo Nordisk Pharma Ltd., Sumitomo Pharm and MSD; research funding from Sysmex, and Nissui; and subsidies or donations from Novartis Pharma, Sanofi K.K., and Novo Nordisk Pharma. Hideaki Miyoshi has received honoraria from Astellas Pharma, Eli Lilly Japan, MSD, Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., and Taisho Pharmaceutical Co; research funding from Mitsubishi Tanabe Pharma Co. and Taisho Pharmaceutical Co.; and subsidies or donations from Ono Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Abbott Japan Co., Taisho Pharmaceutical Co., Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., and LifeScan Japan Inc. Daisuke Yabe has received consulting or speaker fees from Astellas Pharma, Eli Lilly Japan, MSD, Novo Nordisk Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceuticals, Sumitomo Dainippon Pharma, and Takeda Pharmaceutical; and clinically commissioned/joint research grants from Ono Pharmaceuticals, Novo Nordisk Pharma, Taisho Pharmaceutical, Arklay, and Terumo. Yoko Takahashi and Yukiko Morimoto are employees of Sanofi K.K and may hold shares and/or stock options in the company. Yasuo Terauchi has received honoraria for serving on advisory boards for MSD, Boehringer Ingelheim, Tanabe-Mitsubishi, Daiichi Sankyo, Novo Nordisk, Eli Lilly, Sanofi K.K., Astellas Pharma, and AstraZeneca; honoraria for lectures from MSD, Ono Pharmaceuticals, Boehringer Ingelheim, Takeda, Tanabe-Mitsubishi, Daiichi Sankyo, Sanwa Kagaku Kenkyusho, Novo Nordisk, Eli Lilly, Sanofi K.K., Dainippon-Sumitomo, Shionogi, Bayer Yakuhin, Astellas and AstraZeneca; and research funding from MSD, Ono Pharmaceuticals, Boehringer Ingelheim, Novartis, Takeda, Daiichi Sankyo, Novo Nordisk, Eli Lilly, Sanofi K.K., and Dainippon-Sumitomo.
Compliance with Ethics Guidelines
Anonymized individual-level data were retrospectively collected for this study; individuals provided written consent for their data to be used. Ethics committee approval for the study was applied for and granted prior to study initiation by a central ethics committee (Sone Clinic in Tokyo, Japan; approval granted 24 February 2021) for some institutions, and by their own institutional ethics committee in other institutions (Supplementary Table S1).
Data Availability
Qualified researchers may request access to participant-level data and related documents. Participant-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at http://www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsuhisa, M., Miyoshi, H., Yabe, D. et al. Use of iGlarLixi for Management of Type 2 Diabetes in Japanese Clinical Practice: SPARTA Japan, a Retrospective Observational Study. Diabetes Ther 14, 219–236 (2023). https://doi.org/10.1007/s13300-022-01333-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01333-w