Abstract
Introduction
Liraglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), is effective in patients with type 2 diabetes (T2D), but treatment discontinuation without new T2D therapy initiation may compromise outcomes.
Methods
This retrospective cohort study (July 1, 2012, to December 31, 2019) identified patients ≥ 18 years with T2D in the Optum® Clinformatics® Data Mart who discontinued liraglutide (index date). Patients with continuous enrollment for ≥ 12 months before and after discontinuation (baseline), ≥ 6 months liraglutide coverage pre-index, and no new T2D therapy start during follow-up were included. Changes from baseline in all-cause healthcare resource utilization (HCRU; outpatient visits, emergency room [ER] visits, and hospitalization events), costs, and glycated hemoglobin (HbA1c) over 12 months after discontinuation were evaluated.
Results
Overall, 625 of 186,630 patients who discontinued liraglutide during the baseline period (mean [standard deviation (SD)] age, 62.1 [10.1] years) were included in the 12-month analysis. A significant increase in the rate of ER visits (rate ratio [95% confidence interval (CI)]: 1.23 per 100 person-months [1.05, 1.43]; P = 0.0079), hospitalizations (1.36 [1.09, 1.70]; P = 0.0056), and outpatient visits (1.03 [1.01, 1.06]; P = 0.0075) was observed. Total HCRU costs significantly increased after discontinuation ($436.12 per patient per month [$90.07, $782.17]; P = 0.0136), driven by significantly higher outpatient costs ($238.70 [$34.16, $443.25]; P = 0.0223). HbA1c increased significantly by 12 months from mean (SD) 7.37 (1.53) at baseline to 7.63 (1.64; difference: + 0.25 [95% CI 0.14, 0.36]; P < 0.0001).
Conclusions
Patients who discontinued liraglutide showed increases in HCRU; costs, mainly driven by outpatient cost; and HbA1c within 12 months, emphasizing the importance of treatment optimization on clinical and economic outcomes in patients with T2D.
Plain Language Summary
Liraglutide is indicated in patients with type 2 diabetes and elevated cardiovascular risk or established cardiovascular disease. In this study, we observed that adults with type 2 diabetes who discontinued liraglutide showed increases in healthcare resource utilization; costs, which were mainly driven by increases in outpatient visits; and glycated hemoglobin within 12 months. In adults with type 2 diabetes, treatment discontinuation without restarting or initiating a new therapy impacts short-term healthcare resource utilization and economic outcomes, and future work may further investigate the long-term implications of sustained treatment discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Delayed initiation of treatment and treatment discontinuation are common in patients with T2D and may be attributable in part to clinical inertia, which substantially contributes to the clinical and economic burden associated with T2D in the US. |
A better understanding of the clinical and economic impact of treatment discontinuation among these patients can help support and inform multi-stakeholder programs dedicated to the continuity of care for patients with T2D, particularly those with an elevated risk of cardiovascular disease or other complications. |
This study evaluated changes in HCRU, costs, and HbA1c over 12 months after discontinuation of liraglutide among adults who did not initiate another modern antidiabetic or insulin therapy. |
Post-discontinuation, a significant increase in the rate of emergency room visits, hospitalizations, and outpatient visits was observed over the 12-month follow-up. Total HCRU costs were significantly higher 12 months after discontinuation, mainly driven by significant outpatient costs. HbA1c increased significantly at 12 months from baseline. |
Discontinuation of therapy without restarting or initiating a new therapy impacts short-term clinical and economic outcomes for adults with T2D in the US. |
Introduction
According to the 2018 American Diabetes Association (ADA) estimates, approximately 34.2 million Americans have diabetes [1], of whom 90–95% have type 2 diabetes (T2D) [2]. T2D and related complications are known to incur a meaningful clinical and humanistic burden on patients [3, 4], with a substantial economic burden on public and private payers in the United States (US) [5, 6]. For diagnosed patients, total costs associated with diabetes are estimated to be $327 billion (2017 estimates), with $237 billion attributed to direct medical costs and $90 billion to reduced productivity [1, 2]. The prevalence and burden of T2D among Americans ≥ 65 years of age, whether diagnosed or undiagnosed, are particularly high (26.8% or 14.3 million seniors) [1, 3].
As the economic burden of T2D increases with related complications, improving glycemic control to prevent complications is an important clinical goal that can also help reduce costs [7, 8]. Reduction in glycated hemoglobin (HbA1c) of 1% is associated with reductions in all-cause and diabetes-related total healthcare costs, resulting in annual cost savings of $429 and $736 per patient, respectively [9]. Reducing T2D complications reduces costs by $67 to $105 per patient per month (PPPM) among commercial plan beneficiaries and by $99 to $158 PPPM among Medicare beneficiaries [3].
Modern non-insulin antidiabetic drugs (NIADs), such as injectable glucagon-like peptide-1 receptor agonists (GLP-1 RAs), have demonstrated efficacy, safety, and cost-effectiveness in patients with T2D [10,11,12]. Liraglutide is recommended as an adjunct to diet and exercise to improve glycemic control and to reduce the risk of major adverse cardiovascular events in adults with T2D and established cardiovascular disease [13]. Liraglutide is a cost-effective and budget-neutral treatment option for T2D from a US third-party payer perspective [14, 15].
When effective therapeutic options are available, supporting appropriate treatment persistence and adherence is important for achieving optimal outcomes related to glycemic control and reducing long-term complications, mortality, and subsequent healthcare resource utilization (HCRU) and costs [16, 17]. However, the discontinuation rate for injectable GLP-1 RAs in US clinical practice exceeds 47% after 12 months and 70% after 24 months in patients with T2D [18]. Numerous factors influence T2D medication adherence (such as perceived efficacy, occurrence of hypoglycemia, treatment complexity, convenience, and treatment costs) and persistence (such as medication class, sex, younger age, and non-White ethnicity) [19,20,21,22]. Delayed initiation of treatment and treatment discontinuation are common in routine clinical care for patients with T2D and may be attributable in part to clinical inertia, which makes a substantial contribution to the clinical and economic burden associated with T2D in the US [23].
A better understanding of the clinical and economic impact of treatment discontinuation among these patients can help support and inform multi-stakeholder programs dedicated to the continuity of care for patients with T2D, particularly those with an elevated risk of cardiovascular disease or other complications. In this study, we aimed to evaluate changes in HCRU, costs, and HbA1c over 12 months after discontinuation of liraglutide among adults who did not initiate another modern NIAD or insulin. A subgroup analysis including patients aged ≥ 65 years and those with 24 months of follow-up was also performed.
Methods
This retrospective, non-interventional cohort study evaluated 12-month changes in all-cause HCRU and costs among adults (≥ 18 years old) with T2D in the Optum® Clinformatics® Data Mart after discontinuation of liraglutide between July 1, 2012, and December 31, 2019. The Optum Clinformatics Data Mart is derived from a database of medical and pharmacy administrative claims for members of large commercial and Medicare Advantage health plans, including approximately 17–19 million annual covered lives. The Clinformatics Data Mart is statistically de-identified under the Expert Determination method, consistent with the Health Insurance Portability and Accountability Act (HIPAA), and managed according to Optum customer data use agreements (Optum, Inc., Eden Prairie, MN; www.optum.com) [24]. The population is geographically diverse and spans all 50 US states. Optum standard pricing algorithms create standard prices that reflect the intensity of care provided, where differences in price amount to differences in utilization in the de-identified claims data. The payment amounts (insurance payment plus the patient payment) in the pricing algorithm account for the number of services provided, relative resource costs, and nature of healthcare utilization. Inpatient evaluations in the algorithm are based on the Medicare Severity Diagnostic Related Group (DRG), while outpatient services are based on the Centers for Medicare and Medicaid Services (CMS) Outpatient Prospective Payment System. Lastly, pharmacy costs in the algorithm are based on First Databank (www.fdbhealth.com) [25] and adjusted according to therapeutic category and generic indicator. This study was conducted using secondary data from a large commercial health plan. The data were de-identified; therefore, there was no way to trace personal health information to any individuals in the study. As a result, Institutional Review Board (IRB) approval was not needed. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Patient Population
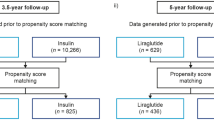
Adults with T2D who discontinued liraglutide during the study period were identified; the date of liraglutide discontinuation was set as the index date with a 12-month pre-index (baseline) period. Patients ≥ 18 years of age with ≥ 2 separate diagnoses of T2D on different days and ≥ 6 months of liraglutide medication coverage during the baseline period (defined as total days’ supply dispensed; consecutive fills not required) were included. Discontinuation of liraglutide was defined as the absence of a refill for ≥ 180 days after exhaustion of days’ supply. All eligible patients were to have continuous health plan coverage for 12 months prior to the index date and throughout the 12-month follow-up period. The HCRU analysis measured changes in HbA1c where eligible patients had HbA1c data available within 90 days prior to the index date and within 90 days after the end of the follow-up period (Fig. 1). The list of drug codes is presented in supplementary information, Table S1.
In order to appropriately evaluate the impact of discontinuing liraglutide therapy without replacement therapy, patients with a new prescription for a modern NIAD or insulin after liraglutide discontinuation that was not observed during the baseline period were excluded (NIADs included sodium-glucose cotransporter 2 [SGLT2] inhibitors, dipeptidyl-peptidase 4 [DPP4] inhibitors, GLP-1 RAs, and thiazolidinediones [TZD]). Exclusions also considered a 90-day window for starting a modern NIAD or insulin prior to the index date, known as a “proximate switching” approach, as the start of a new therapy may have overlapped with the exhaustion of days’ supply for liraglutide. Patients with missing demographic information, evidence of coordination of benefits, pregnancy at any time point during the baseline or follow-up periods, or evidence of bariatric surgery or hospice care during the baseline or follow-up periods were excluded.
Study Variables and Analysis
The study’s primary objective was to evaluate changes from baseline to 12 months in all-cause HCRU and costs, including those for outpatient visits, emergency room (ER) visits, and hospitalizations. Patient demographics and clinical characteristics were evaluated at the index date and reported as frequencies (percentages) for categorical variables and means with standard deviations (SDs) for continuous variables. Outpatient visits included any patient and healthcare provider interaction resulting in a charge, whether office-based, home visit, or virtual. Changes in HbA1c after discontinuation of liraglutide were also measured among those with baseline and follow-up HbA1c values. The average costs PPPM were computed, and the differences between baseline and 12-month values were compared.
A subgroup analysis was performed among adults ≥ 65 years of age for all outcomes. Secondary analyses were also conducted using a 24-month follow-up period (all adults and ≥ 65-year subgroup) and among patients who did not have both baseline and follow-up HbA1c values (12- and 24-month changes in all adults).
Incidence rates for each type of HCRU service (outpatient visits, ER visits, and hospitalizations) were calculated for baseline and follow-up periods based on the count of events divided by the duration. Poisson test was used to calculate and test incidence rate ratios between time periods. Paired t tests were used to evaluate pre- versus post-discontinuation differences in outcomes, with the hypothesis that there would be no difference at an alpha level of 0.05. All analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Primary Analysis: 12-Month Changes in HCRU, Costs, and HbA1c
A total of 186,630 adults with T2D who discontinued liraglutide during the baseline period were identified, of whom 625 patients were eligible for the 12-month primary analysis (Fig. 2). The mean (SD) age of the primary analysis cohort was 62.1 (10.1) years, 51% were women, and approximately half (45%) of all patients had a Charlson Comorbidity Index (CCI) score ≥ 3 (Table 1).
A significant increase in the rate of ER visits (rate ratio [95% confidence interval (CI)]: 1.23 per 100 person-months [1.05, 1.43]; P = 0.0079), hospitalizations (1.36 [1.09, 1.70]; P = 0.0056), and outpatient visits (1.03 [1.01, 1.06]; P = 0.0075) was observed over the 12-month follow-up after liraglutide discontinuation (Table 2). Total all-cause medical costs were significantly higher 12 months after discontinuation of liraglutide ($436.12 PPPM [$90.07, $782.17]; P = 0.0136), driven by significantly higher outpatient visit costs ($238.70 [$34.16, $443.25]; P = 0.0223; Table 2). Increases in hospitalization costs ($126.44 [95% CI, $ − 87.66, $340.54]; P = 0.2466) and ER visit costs ($70.97 [95% CI, $ − 17.09, $159.04]; P = 0.1140) were not significant (Table 2). At the 12-month follow-up, a significant increase in HbA1c (from mean [SD] 7.37 [1.53] to 7.63 [1.64]) was observed versus baseline (difference: + 0.25 [95% CI: 0.14, 0.36]; P < 0.0001).
Subgroup Analysis: 12-Month Changes in HCRU, Costs, and HbA1c in the ≥ 65-Year Subgroup
A total of 288 patients were aged ≥ 65 years and eligible for the 12-month analysis. The mean (SD) age of the ≥ 65-year subgroup was 71.1 (4.56) years, 55% were women, and approximately half (57%) of all patients had a CCI score ≥ 3 (supplementary information, Table S2). A significant increase in the rate of ER visits (rate ratio [95% CI]: 1.31 per 100 person-months [1.02, 1.69]; P = 0.0344), hospitalizations (1.80 [1.29, 2.51]; P = 0.0003), and outpatient visits (1.05 [1.02, 1.09]; P = 0.0022) was observed over the 12-month follow-up after liraglutide discontinuation compared with baseline (Table 3).
Total medical costs over the 12-month follow-up after liraglutide discontinuation significantly increased by $837.34 PPPM (95% CI, $245.99, $1428.69; P = 0.0057) compared with baseline, also driven by increased outpatient visit costs ($485.16 [95% CI, $119.15, $851.17]; P = 0.0096). Increases in hospitalization costs ($228.67 [95% CI, $ − 94.37, $551.71]; P = 0.1646) and ER visit costs ($123.50 [95% CI, $ − 58.89, $305.90]; P = 0.1837) were not significant (Table 3). At the 12-month follow-up, the change in HbA1c from baseline (from mean [SD] 7.37 [1.43] to 7.46 [1.37]) was not significant (difference: 0.09 [95% CI: − 0.05, 0.24]; P = 0.1921).
Secondary Analyses: 24-Month Changes in the Overall Cohort and ≥ 65-Year Subgroup
A total of 237 adults were included in the overall 24-month follow-up cohort (supplementary information, Figure S1). The mean (SD) age of the 24-month follow-up cohort was 62.3 (10.6) years, of whom 45% were women and 46% had a CCI score ≥ 3 (supplementary information, Table S3). A significant increase in the rate of outpatient visits (rate ratio [95% CI]: 1.06 per 100 person-months [1.03, 1.10]; P = 0.0003) was observed over the 24-month follow-up after liraglutide discontinuation compared with baseline (supplementary information, Table S4). Total medical costs over the 24-month follow-up after liraglutide discontinuation significantly increased by $435.89 PPPM (95% CI, $9.41, $862.37; P = 0.0452) compared with baseline. As in the primary analysis, the major contributor to the increase in total costs was the increase in outpatient visit costs ($316.21 [95% CI, $76.27, $556.15]; P = 0.01). Increases in hospitalization costs ($84.72 [95% CI, $ − 194.92, $364.37]; P = 0.5512) and ER visit costs ($34.96 [95% CI, $ − 38.78, $108.70]; P = 0.3513) were not significant (supplementary information, Table S5). At the 24-month follow-up, the change in HbA1c from baseline (from mean [SD] 7.42 [1.66] to 7.47 [1.46]) was not significant (difference: 0.06 [95% CI: − 0.12, 0.23]; P = 0.5327).
A total of 107 adults ≥ 65 years old were included in the 24-month follow-up subgroup analysis. Demographic and clinical characteristics were generally comparable to those of the 12-month ≥ 65-year subgroup (supplementary information, Table S3). A significant increase was observed in the rate of ER visits (rate ratio: 1.48 per 100 person-months [1.02, 2.17]; P = 0.0347) over the 24-month follow-up after liraglutide discontinuation compared with baseline (supplementary information, Table S4). Total medical costs over the 24-month follow-up after liraglutide discontinuation increased by $553.60 PPPM (95% CI, $ − 211.26, $1318.46; P = 0.1542) compared with baseline. Increases in costs related to outpatient visits, hospitalizations, and ER visits were not statistically significant (supplementary information, Table S5). At the 24-month follow-up, the change in HbA1c from baseline (mean [SD] 7.33 [1.57] to 7.21 [1.15]) was not significant (difference: − 0.12 [95% CI: − 0.38, 0.14]; P = 0.3555).
Secondary Analyses: 12- and 24-Month Changes in Patients Missing the HbA1c Data Criteria
After removing the baseline and follow-up HbA1c data requirement, the broader 12-month secondary analysis cohort comprised 6,674 patients (Fig. 2). Demographic and clinical characteristics were generally comparable to those of the primary analysis cohort (supplementary information, Table S6). A statistically significant increase in the rate of ER visits (rate ratio [95% CI]: 1.08 per 100 person-months [1.04, 1.12]; P = 0.0001), hospitalizations (1.24 [1.16, 1.33]; P < 0.0001), and outpatient visits (1.01 [1.01, 1.02]; P = 0.0005) was observed over the 12-month follow-up after liraglutide discontinuation compared with baseline (supplementary information, Table S7). The total cost over the 12-month follow-up after liraglutide discontinuation significantly increased by $364.21 PPPM (95% CI, $261.58, $466.83; P < 0.0001). Costs for outpatient visits, hospitalizations, and ER visits were all significantly higher after 12 months compared with baseline (all P < 0.001; supplementary information, Table S8).
After removing the HbA1c eligibility criteria, the 24-month secondary analysis cohort comprised 2909 patients (supplementary information, Figure S1). Overall, 41.9% of patients were ≥ 65 years of age and 48.1% were male (supplementary information, Table S6). A significant increase in the rate of ER visits (rate ratio [95% CI]: 1.16 per 100 person-months [1.10, 1.23]; P < 0.0001) and a significant decrease in outpatient visits (0.98 [0.97, 0.99]; P = 0.0006) were observed over the 24-month follow-up after liraglutide discontinuation compared with baseline. The increase in the rate of hospitalizations (1.09 per 100 person-months [0.99, 1.20]; P = 0.0713) was not significant (supplementary information, Table S7). Total medical costs over the 24-month follow-up after liraglutide discontinuation significantly increased by $256.89 PPPM (95% CI, $126.26, $387.51; P = 0.0001). The major contributor to the increase in total costs was the significant increase in outpatient visit costs ($205.49 [95% CI, $114.61, $296.37]; P < 0.0001). Increases in hospitalization costs ($43.36 [95% CI, $ − 24.12, $110.85]; P = 0.2078) and ER visit costs ($8.03 [95% CI, $ − 28.77, $44.83]; P = 0.6687) were not significant (supplementary information, Table S8).
Discussion
This study demonstrated significant increases in HCRU, costs, and HbA1c over 12 months after adults with T2D discontinued liraglutide without restarting or initiating new treatment. Increases in HCRU were statistically significant for outpatient visits, ER visits, and hospitalizations. Significant increases in total all-cause medical costs appeared to be driven by the increased costs of outpatient visits. A statistically significant increase in HbA1c was also observed after 12 months in this insured adult cohort; however, the increase was not significant at the 24-month follow-up. Results related to HCRU and costs were generally consistent in a subgroup analysis of patients ≥ 65 years of age and secondary analyses accounting for a 24-month follow-up period and absence of HbA1c values at both baseline and follow-up.
A significant increase in HCRU was observed in patients ≥ 65 years of age over the 12-month follow-up after liraglutide discontinuation, with almost double the increase in cost compared with the overall cohort. However, over the 24-month follow-up, a significant increase was observed only in ER visits, suggesting that discontinuation and disease progression in patients ≥ 65 years of age may have led to more serious complications/comorbidities requiring ER visits. Additional HCRU from discontinuing liraglutide in patients ≥ 65 years of age was not reflected in HbA1c levels at the 12- and 24-month follow-ups. Stopping a therapy for whatever reason without an appropriate treatment plan can have implications for the health of the patient and cost to the system. Given the different drug classes, routes of administration, and financial assistance programs, no patient should be allowed to fall through the cracks. Although we did not evaluate the reasons for discontinuing liraglutide, non-adherence/non-persistence negatively impacts the successful outcome, and this is especially true in the diabetes space [18]. Non-adherence to oral antidiabetic medications has been associated with increased risk of hospitalization, greater hospitalization costs, and longer hospital stays, as reported in State Pharmaceutical Assistance Program (SPAP)-enrolled elderly patients. Medication non-adherence was associated with significantly greater all-cause ($22,670 vs. $16,383) and diabetes-related ($13,518 vs, $12,634) hospitalization costs [26].
Findings from the secondary analysis in patients without baseline and follow-up HbA1c data were consistent with the results of the 12-month primary analysis, with a significant increase in HCRU and associated costs after liraglutide discontinuation. In the 24-month follow-up cohort, a significant increase in ER visits, total costs, and outpatient visit costs was observed. Over 24 months, patients also had a slight increase in HbA1c levels and rates of HCRU events, suggesting that treatment discontinuation of this duration may be costly. Patients in this study were reasonably controlled on liraglutide at baseline based on the ADA guidelines [27, 28], but their HbA1c levels started increasing within 12 months of discontinuation, emphasizing the importance of staying on therapy. As an adjunct treatment, liraglutide can help achieve the target ADA guideline [27] recommendations in adults to lower HbA1c levels without significant hypoglycemia or other adverse effects based on provider judgment and patient preference, or less-stringent HbA1c goals in appropriate patients [28]. Short-term outcomes observed in this study from discontinuing treatment without restarting or replacing it began to show an impact on glycemic control and subsequent requirements for physician encounters, as well as ER visits and hospitalizations associated with more urgent and serious care requirements. This was most evident in the secondary analysis that did not require the presence of HbA1c values in the database, which may have approximated a more authentic real-world population of patients seen in US clinical practice (since a quantitative limitation was removed).
Strengths and Limitations
This study should be interpreted in the context of certain strengths and limitations. We used a large, geographically diverse administrative claims database to investigate real-world outcomes in an insured US population. The primary and secondary analyses were generally consistent and may offer generalizability to insured US adults receiving injectable GLP-1 therapy nationwide. However, as the study sample was from an administrative claims database, and represents people insured with United Healthcare, the T2D population selected for the study may not reflect the general T2D population. Medication use was identified via evidence of prescription fill dates; however, the investigators did not have access to information detailing whether patients took their medications as prescribed. We did not assess the potential dose modifications of concomitant baseline medications (especially for insulin usage, which was high in this population) in defining treatment modification, which may impact the results. We accounted for medical reasons for treatment discontinuation by excluding patients with bariatric surgery or hospice care during the study period, though other medical reasons not visible in the data source may have been present. Administrative coding errors are possible and cannot be ruled out, creating possible selection bias or misclassification bias. To reduce selection or misclassification bias, the study required at least two separate diagnoses of T2D on different days so as to reduce the error of a misdiagnosis for T2D.
The study data reflect Optum-imputed costs rather than original paid costs. In addition, as the cost of metformin is low, some patients pay out-of-pocket rather than through insurance; thus, all use of metformin may not have been captured in the database.
Conclusions
Adults with T2D who discontinued liraglutide showed increases in all-cause HCRU, costs, and HbA1c within 12 months, driven by increases in outpatient visits. Increased HCRU and costs were also observed among those with 24 months of follow-up. Findings were consistent in the secondary analysis looking at the overall population and in a subgroup of patients ≥ 65 years of age. However, the significant increase in HCRU observed in patients aged ≥ 65 years over the 12-month follow-up after liraglutide discontinuation was almost double the increase in cost compared with the overall cohort. Discontinuation of therapy without restarting or initiating a new therapy impacts short-term clinical and economic outcomes, and future work may further investigate the long-term implications of treatment discontinuation without replacement for adults with T2D in the US.
References
American Diabetes Association. Statistics about diabetes. 2021. https://www.diabetes.org/resources/statistics/statistics-about-diabetes. Accessed 10 Oct 2021.
Dougherty T, Heile M. Type 2 diabetes in the US managed care setting: the burden of disease and rationale for an oral glucagon-like peptide-1 receptor agonist. Am J Manag Care. 2020;26:S325–34.
Fitch K, Pyenson BS, Iwasaki K. Medical claim cost impact of improved diabetes control for Medicare and commercially insured patients with type 2 diabetes. J Manag Care Pharm. 2013;19:609–20.
Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with type 2 diabetes in U.S. managed care health plans: results from Translating Research Into Action for Diabetes (TRIAD). Diabetes Care. 2012;35:2250–6.
Candrilli SD, Meyers JL, Boye K, Bae JP. Health care resource utilization and costs during episodes of care for type 2 diabetes mellitus-related comorbidities. J Diabetes Compl. 2015;29:529–33.
Ramzan S, Timmins P, Hasan SS, Babar ZU. Cost analysis of type 2 diabetes mellitus treatment in economically developed countries. Expert Rev Pharmacoecon Outcomes Res. 2019;19:5–14.
Caro JJ, Ward AJ, Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–81.
Minshall ME, Roze S, Palmer AJ, et al. Treating diabetes to accepted standards of care: a 10-year projection of the estimated economic and health impact in patients with type 1 and type 2 diabetes mellitus in the United States. Clin Ther. 2005;27:940–50.
Lage MJ, Boye KS. The relationship between HbA1c reduction and healthcare costs among patients with type 2 diabetes: evidence from a U.S. claims database. Curr Med Res Opin. 2020;36:1441–7.
Morieri ML, Rigato M, Frison V, et al. Effectiveness of dulaglutide vs liraglutide and exenatide once-weekly. A real-world study and meta-analysis of observational studies. Metabolism. 2020;106:154190.
Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes Metab. 2019;21:920–9.
Li Q, Ganguly R, Ganz ML, Gamble C, Dang-Tan T. Real-world clinical effectiveness and cost savings of liraglutide versus sitagliptin in treating type 2 diabetes for 1 and 2 years. Diabetes Ther. 2018;9:1279–93.
Victoza [prescribing information]. Novo Nordisk A/S, August 25, 2017. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf. Accessed 10 Oct 2021.
Shah D, Risebrough NA, Perdrizet J, Iyer NN, Gamble C, Dang-Tan T. Cost-effectiveness and budget impact of liraglutide in type 2 diabetes patients with elevated cardiovascular risk: a US-managed care perspective. Clinicoecon Outcomes Res. 2018;10:791–803.
Zueger PM, Schultz NM, Lee TA. Cost effectiveness of liraglutide in type II diabetes: a systematic review. Pharmacoeconomics. 2014;32:1079–91.
Guerci B, Chanan N, Kaur S, Jasso-Mosqueda JG, Lew E. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10:437–49.
Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabetes Med. 2015;32:725–37.
Weiss T, Carr RD, Pal S, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adher. 2020;14:2337–45.
McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–3.
Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29:1275–86.
Horii T, Momo K, Yasu T, Kabeya Y, Atsuda K. Determination of factors affecting medication adherence in type 2 diabetes mellitus patients using a nationwide claim-based database in Japan. PLoS ONE. 2019;14: e0223431.
Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adher. 2016;10:1299–307.
Ali SN, Dang-Tan T, Valentine WJ, Hansen BB. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. 2020;37:869–82.
Optum, Inc. 2021. http://www.optum.com. Accessed 10 Oct 2021.
First Databank. Drug Database and Medical Device Database Leader. 2021. http://www.fdbhealth.com. Accessed 10 Oct 2021.
Pednekar P, Heller DA, Peterson AM. Association of medication adherence with hospital utilization and costs among elderly with diabetes enrolled in a state pharmaceutical assistance program. J Manag Care Spec Pharm. 2020;26:1099–108.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S66–76.
American Diabetes Association. 12. Older adults: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S152–62.
Acknowledgements
Funding
The study, including the Rapid Service Fee, was sponsored by Novo Nordisk, Inc.
Medical Writing, Editorial, and Other Assistance
Editorial support, in the form of medical writing, assembling tables, and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing, was provided by Annirudha Chillar, MD, PhD, of Cactus Life Sciences (part of Cactus Communications) and funded by NNI.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Josh Noone; Formal analysis and investigation: Yuanjie Liang; Methodology, interpretation of results and manuscript writing: Chioma Uzoigwe, Josh Noone, Yuanjie Liang, Sarah Naz Ali, Cory Gamble. All authors have read and approved the final version of the manuscript.
Disclosures
Chioma Uzoigwe, Cory Gamble, and Josh Noone are salaried employees and shareholders of Novo Nordisk. Yuanjie Liang is a salaried employee of Novo Nordisk. Sarah Naz Ali was a salaried employee of Novo Nordisk during the conduct of this study and is currently an employee of Otsuka Pharmaceutical Development and Commercialization.
Compliance with Ethics Guidelines
This study was conducted using secondary data from a large commercial health plan. The data was de-identified; therefore, there was no way to trace personal health information to any individuals in the study. Therefore, Institutional Review Board (IRB) approval was not needed. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Uzoigwe, C., Noone, J., Liang, Y. et al. Increased Healthcare Resource Use and Costs After Discontinuation of Liraglutide in Patients with Type 2 Diabetes from a Commercial- and Medicaid-Insured Claims Database. Diabetes Ther 13, 1861–1874 (2022). https://doi.org/10.1007/s13300-022-01322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01322-z