Abstract
Over recent years, the expanding evidence base for sodium-glucose cotransporter-2 inhibitor (SGLT2i) therapies has revealed benefits beyond their glucose-lowering efficacy in the treatment of Type 2 diabetes mellitus (T2DM), resulting in their recognition as cardiorenal medicines. While SGLT2is continue to be recommended among the second-line therapies for the treatment of hyperglycaemia, their true value now extends to the prevention of debilitating and costly cardiovascular and renal events for high-risk individuals, with particular benefit shown in reducing major adverse cardiac events and heart failure (HF) and slowing the progression of chronic kidney disease. However, SGLT2i usage is still suboptimal among groups considered to be at greatest risk of cardiorenal complications. The ongoing coronavirus disease 2019 (COVID-19) pandemic has intensified financial pressures on healthcare systems, which may hamper further investment in newer effective medicines. Emerging evidence indicates that glycaemic control should be prioritised for people with T2DM in the era of COVID-19 and practical advice on the use of T2DM medications during periods of acute illness remains important, particularly for healthcare professionals working in primary care who face multiple competing priorities. This article provides the latest update from the Improving Diabetes Steering Committee, including perspectives on the value of SGLT2is as cost-effective therapies within the T2DM treatment paradigm, with particular focus on the latest published evidence relating to the prevention or slowing of cardiorenal complications. The implications for ongoing and future approaches to diabetes care are considered in the light of the continuing coronavirus pandemic, and relevant aspects of international treatment guidelines are highlighted with practical advice on the appropriate use of SGLT2is in commonly occurring T2DM clinical scenarios. The ‘SGLT2i Prescribing Tool for T2DM Management’, previously published by the Steering Committee, has been updated to reflect the latest evidence and is provided in the Supplementary Materials to help support clinicians delivering T2DM care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Improving Diabetes Steering Committee has reviewed the wealth of evidence supporting the role of sodium-glucose cotransporter-2 inhibitor (SGLT2i) therapies as cardiorenal and glucose-lowering medicines used in the management of Type 2 diabetes mellitus (T2DM). |
Data from randomised cardiovascular outcomes trials and real-world studies have demonstrated the value of SGLT2i therapies in reducing cardiorenal events, with particular benefit shown regarding the slowing of disease progression in chronic kidney disease (CKD) and diabetic kidney disease as well as the avoidance of heart failure events. |
The proven cost-effectiveness profile for SGLT2i therapies (previously based purely upon their glucose-lowering efficacy) has been strengthened by evidence from recent SGLT2i cardiovascular and renal outcome trials, in which costly adverse outcomes were reduced and subsequent improvements in quality of life were provided for people with T2DM. |
The ongoing coronavirus disease 2019 (COVID-19) pandemic has heightened the challenges of T2DM management for many healthcare professionals (HCPs) and people living with the disease. The threat of severe COVID-19 infection emphasises the importance of maintaining good glycaemic control for people with diabetes. In this context, as clinically efficacious and cost-effective medicines, SGLT2i therapies can help people with T2DM reach their glycaemic targets. |
As the evidence base for SGLT2i therapies continues to broaden and multiple treatment guidelines are adapted to reflect the latest data, HCPs may benefit from practical advice on the appropriate place of these medicines within the T2DM treatment pathway. The ‘SGLT2i Prescribing Tool for T2DM Management’, previously published by the Steering Committee, has been revised and developed further to provide a quick reference guide that aims to support HCPs working in the field of T2DM management and to encourage the appropriate use of SGLT2is in clinical practice. |
The Role of the Improving Diabetes Steering Committee
The Improving Diabetes Steering Committee was formed in 2017 and comprises a panel of clinical experts from across primary and specialist care who have worked alongside prominent professional organisations to improve the delivery of diabetes care. The Committee’s initial aim was to support healthcare professionals (HCPs) working in Type 2 diabetes mellitus (T2DM) medicine within the UK. However, the panel has recently welcomed European experts to the group, bringing new insights regarding the shared challenges faced by HCPs across Europe in T2DM management. The Committee is supported by an educational grant from Napp Pharmaceuticals Limited and Mundipharma Research Limited and endeavours to ensure that HCPs who prescribe diabetes medicines have access to balanced and accurate information and evidence concerning T2DM medicines, with a specific focus on the sodium-glucose cotransporter- 2 inhibitor (SGLT2i) class. The group is committed to providing healthcare colleagues with clarity regarding the evidence base supporting SGLT2i agents and appropriate approaches to prescribing. Educational materials and publications provided by the panel, such as the previously published consensus documents, are intended to increase confidence and understanding regarding the correct place of these medicines within the current T2DM treatment pathway [1,2,3].
This narrative review paper summarises the key topics that were discussed during a Steering Committee meeting held in July 2021 and aims to examine the accumulating evidence surrounding the cardiorenal benefits of SGLT2i medicines. The true value of these therapies in T2DM management is considered as well as the practicalities of prescribing SGLT2is in modern clinical practice, with emerging insights drawn from the era of the coronavirus disease 2019 (COVID-19) pandemic.
This article is based upon previously conducted studies and does not involve any new studies of human or animal subjects. Some authors were involved in studies discussed within the paper that included human subjects, all of which complied with the tenets of the Declaration of Helsinki of 1964 and subsequent revisions and used protocols that had been approved by relevant Institutional Review Boards/Ethics Committees and included patients who had provided written informed consent.
The Clinical Effects of SGLT2i Therapies: From Glucose-Lowering to Cardiorenal Protection
Although initially approved as medicines for the treatment of hyperglycaemia in people with T2DM, the vast and rapidly developing evidence base for SGLT2i medicines has driven a paradigm shift in clinical perceptions regarding their place within the therapeutic pathway [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. In addition to their proven efficacy in reducing hyperglycaemia, numerous large-scale randomised clinical trials (RCTs), real-world studies, systematic reviews and meta-analyses have revealed SGLT2i therapies to be effective cardiorenal medicines [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The protective effects of SGLT2is against the cardiovascular (CV) and renal complications of T2DM appear to be independent of their glucose-lowering activity, with particular value being demonstrated in the reduction of risk regarding hospitalisation for heart failure (HHF) and progression of diabetic kidney disease (DKD) as well as lowering the incidence of major adverse cardiovascular events (MACE) [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Recently published reviews have described and explored the putative mechanisms underlying the cardiorenal benefits of SGLT2is, emphasising the direct and indirect effects that these medicines have on pathways that mediate inflammation, oxidative stress and endothelial cell dysfunction [37, 38].
Table 1 provides an overview of cardiorenal outcomes from randomised SGLT2i trials alongside the relevant study populations in which efficacy was demonstrated [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36, 39,40,41]. CV outcome trials (CVOTs) examining the treatment effects of empagliflozin, canagliflozin and dapagliflozin, versus placebo, demonstrated significant reductions in CV events, including composite endpoints encompassing MACE (comprising non-fatal myocardial infarction, non-fatal stroke and cardiovascular mortality), HF and CV death, HHF and worsening HF events [4,5,6,7]. The ertugliflozin CVOT (VERTIS-CV) also demonstrated numerical reductions in some CV and renal events, with a significant decrease in HHF events shown [8]. Emerging data in people with HF with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) indicate that the benefits relating to reduction of HF events are relevant to high-risk populations with and without T2DM [8,9,10,11,12,13,14,15]. The protective CV effects of SGLT2is have been reaffirmed in the real-world setting through large observational studies that include the multinational CVD-REAL study, which showed that SGLT2i therapy was associated with a lower risk of HHF or death in people with or without CV disease (CVD), compared with other glucose-lowering therapies [17,18,19,20].
Data from the EMPA-REG Outcome Trial, CANVAS Program and DECLARE-TIMI 58 trial suggested that, compared with placebo, progression of kidney disease was significantly reduced with SGLT2i treatment, and meta-analysis of SGLT2i CVOTs confirmed these outcomes in people with and without atherosclerotic cardiovascular disease (ASCVD) [4,5,6, 21]. The CREDENCE trial was the first randomised SGLT2i study to show benefit in people with albuminuric chronic kidney disease (CKD) and T2DM [22]. Subsequently, the DAPA-CKD trial has shown benefits in CKD populations with and without diabetes [22, 23]. Data from other RCTs, real-world studies, post-hoc analyses and meta-analyses have consistently shown improvements in renal outcomes in people with diabetes across the SGLT2i class [23,24,25,26,27,28,29,30,31,32,33,34,35,36, 39,40,41].
The Use of SGLT2i Therapies in Current Clinical Practice
The latest editions of clinical guidelines and position statements from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD), Kidney Disease: Improving Global Outcomes (KDIGO), the Association for British Clinical Diabetologists (ABCD) and UK Kidney Association (UKKA) and the European Society of Cardiology (ESC) reflect the plethora of published data showing cardiorenal benefit with SGLT2i treatments in people with T2DM [42,43,44,45,46]. Publication of national guidelines may take longer, compared with international recommendations [47]. Modern guidelines typically recommend SGLT2is as a second-line option for T2DM therapy (after metformin), or as first-line therapy in cases where an individual is unable to tolerate metformin, with the aim of slowing CVD and CKD/DKD progression and reducing HF events [42,43,44,45,46]. KDIGO guidelines recommend starting treatment with metformin and SGLT2is for people with T2DM and CKD [43].
Despite the wealth of clinical evidence and guidance surrounding SGLT2i treatments, global and US data indicate that prescribing remains suboptimal for people with T2DM who have increased CV or renal risk [41, 48,49,50,51,52,53]. Country-level prescribing data are highly variable, but prescriptions for SGLT2i therapies are generally < 15% when comparing against other second-line T2DM treatments [41, 48,49,50,51,52,53,54,55]. Sulphonylurea (SU) treatments continue to be the most commonly prescribed second-line therapy, despite studies showing that newer glucose-lowering treatments (SGLT2is, glucagon-like peptide-1 receptor agonists [GLP-1 RAs]) provide improved clinical outcomes when used with metformin, compared with SU–metformin combinations [41, 48,49,50,51,52,53,54,55]. Data from the DISCOVER study showed that people prescribed a newer glucose-lowering treatment alongside metformin demonstrated greater weight loss and fewer hypoglycaemic events than those receiving SU–metformin combination therapy [54]. The same study revealed that, compared with SU–metformin treatment, SGLT2i–metformin combination therapy was associated with improvements in health-related quality of life (QoL) measures over a 36-month follow-up [54].
Variability in local reimbursement arrangements presents a barrier to SGLT2i prescribing, and these medicines have only recently become available in countries such as France, where experience and confidence with SGLT2is may consequently be low [41, 55,56,57]. Some countries (e.g. Italy) require SGLT2i prescriptions to be initiated and reviewed by a specialist secondary care physician, and bureaucratic processes may hinder the use of newer medicines in other locations [55].
Caution in initiating SGLT2i therapies might, in part, be a consequence of the inconsistent approaches used in the reporting of safety outcomes from CVOTs [4,5,6,7,8,9,10,11,12]. The ongoing COVID-19 pandemic has dramatically impacted the delivery of healthcare services, particularly in primary care, with many medical consultations taking place virtually and clinical assessment becoming increasingly challenging. Most primary and secondary care services have struggled to address the current backlog in T2DM reviews and HCPs may feel uncomfortable starting newer classes of drug when face-to-face consultations or opportunities for monitoring are limited. In addition, the sheer number of new T2DM medications becoming available in recent years could have caused reasonable confusion among HCPs regarding the optimal place of each therapy within the ever more complex treatment pathway. Systematic review data show that therapeutic inertia typically increases with treatment intensification in T2DM management [41, 52]. Clinicians may welcome greater clarity and direction to support them in understanding the value that newer treatments can offer and the individuals most appropriate to receive such therapies.
The Value of SGLT2 Inhibitors in T2DM Management
Prior to the publication of outcomes from the CVOTs and the wealth of data that subsequently followed, internationally recognised reimbursement authorities considered the licensed SGLT2i treatments to be cost-effective in the management of T2DM, based upon their glucose-lowering efficacy [58]. It therefore seems reasonable to assume that the value or cost-effectiveness of SGLT2i treatments should have increased given the evidence surrounding their protective cardiorenal effects and the revised licensed indications across the treatment class [59,60,61].
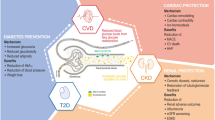
Considerations regarding value in primary care generally focus on drug acquisition cost rather than the long-term clinical advantages of treatments that lower the incidence of cardiorenal events, which are costly to manage and are associated with drastically reduced QoL for people with T2DM. In reality, the true value of a medicine will be represented by many different parameters, from the cost of a treatment through to the avoidance of undesirable clinical outcomes, the impact on QoL and other healthcare costs avoided due to the use of the medicine (e.g. reduced requirement for diabetic retinopathy management where glycated haemoglobin [HbA1c] has been effectively controlled) [62]. Outside of the healthcare system, the broader societal impact of appropriate disease management may include factors such as productivity, absenteeism and presenteeism [62]. Figure 1 provides a summary of the key components of value for pharmacological interventions. While prescribing costs often drive treatment selection and formulary decisions, these represent < 10% of the total healthcare expenditure, and approximately 80% of spending in T2DM management is due to the complications of diabetes, many of which result in hospitalisation and are associated with extended lengths of stay [62,63,64,65,66]. The perceived value for the person with T2DM is also an important aspect of treatment. Interventions that improve QoL and assist in either avoiding or reducing the severity of adverse outcomes (e.g. the need for renal replacement therapy) must be considered of greater worth than treatments that are not able to provide such outcomes or may even be associated with potential harm (e.g. hypoglycaemia and weight gain with SU treatments). Patient-reported outcomes data are limited regarding SGLT2i treatments, although the DAPA-HF trial showed improvements in physical function and QoL relative to usual care in people with HFrEF with/without T2DM and another dapagliflozin study revealed improved treatment satisfaction compared with usual care in people with overweight/obesity and T2DM, with satisfaction significantly correlated to body weight loss [67, 68].
An overview of key drivers of value relating to disease burden and pharmacological interventions [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. The value of a pharmacological intervention encompasses many different parameters relating to the direct cost of treatment, avoidance of resource- and time-consuming adverse events or disease-related complications, the impact on recipient QoL (as well as the burden of illness for family members and those who care for them) and the indirect implications of therapy on wider societal factors. HF Heart failure, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life years, QoL quality of life, RCT randomised controlled trial, RRT renal replacement therapy
Taking a broader view, it seems clear that interventions capable of preventing cardiorenal events offer greater efficiencies within the treatment pathway and that these benefits comfortably off-set prescribing costs. In the UK, the latest National Institute for Health and Care Excellence (NICE) guidelines recognise this concept, stating that (although recommendations relating to the broader prescribing of SGLT2is in clinical practice may carry an associated cost impact) there was likely to be a long-term cost saving through the use of SGLT2is as CKD progression will be slowed and the number of CV and end-stage renal events reduced [47]. HCPs may underestimate the real value of SGLT2is when assessing them simply on their glucose-lowering properties, while ignoring the breadth of data on adverse CV and renal event avoidance [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36, 39,40,41]. Cost-effectiveness analyses suggest that SGLT2i therapies provide economic efficiencies over other T2DM treatments when comparing hospitalisations due to complications in people with and without pre-existing CVD [69,70,71]. Healthcare data indicate that the annual cost of managing each person with HHF could be as high as £3873/€4575, while respective DKD (Stage 3a) and dialysis costs are around £1580/€1866 and £27,270/€32,213 per person [41, 71, 72]. Studies comparing potential expenditure on care show that empagliflozin may offer annual savings of about $1355/€1207 per person over dipeptidyl peptidase 4 inhibitor (DPP-4i) treatments due to reductions in the use of inpatient and outpatient services, shorter length of stay in hospital and fewer hospitalisations and emergency department visits [41, 73, 74]. Yearly cost reductions of approximately US$1989/€1693 per person have been estimated to be associated with the avoidance of renal and CV events, based on outcomes from the canagliflozin CREDENCE study [41, 75]. Economic analysis has also shown positive results regarding dapagliflozin treatment for high-risk individuals with T2DM, based on outcomes from the DECLARE-TIMI 58 trial [76].
A recent study examining the cost of CKD in T2DM showed that, although the cost per person was especially high in those with advanced stages of CKD, direct healthcare expenditure was greatest during the initial phases of kidney disease due to the large number of individuals with T2DM in this group [77]. The study authors concluded that the economic impact of CKD in T2DM could be alleviated by timely and comprehensive approaches to management [77]. Some clinicians have stated the case for earlier use of SGLT2is and other newer glucose-lowering therapies in the T2DM treatment pathway, with the aim of delaying or avoiding CV or renal complications, reducing associated costs and improving long-term QoL for people with T2DM [78, 79]. The KDIGO guidelines reflect this approach, recommending SGLT2i use alongside metformin from the start of treatment in people with diabetes and CKD [43].
Real-world evidence is a critical tool in understanding the true value of pharmacological interventions within healthcare systems, providing additional insights concerning QoL measures, pharmacovigilance data, psychosocial factors, healthcare resource utilisation and treatment adherence and persistence. The Improving Diabetes Steering Committee strongly supports and encourages the use of T2DM registries and local audits to capture these data so that they can inform future economic models and clinical guidelines in T2DM management. The evidence base in this area is already rapidly expanding. The Manchester Community Diabetes Education and Support (CoDES) pilot provides an excellent example of a local project with the potential to influence best practice on a wider scale, due to the efficiencies and improvements in provision of care that were achieved [80]. The pilot demonstrated the benefits of holistic approaches to T2DM management in primary care, with integration of secondary and community care services and prescribing of cardio-protective medicines [80]. Financial modelling was included to understand the potential savings associated with a reduced requirement for specialist referrals and achievement of Quality Outcomes Framework (QOF) targets [80]. Retrospective analyses of real-world SGLT2i data conducted by clinicians in Italy have revealed early improvements in albuminuria, compared with other glucose-lowering therapies, and reductions in the composite endpoint of HbA1c, body weight and systolic blood pressure, versus DPP-4is [81,82,83]. In addition, SGLT2i use was associated with improved cardiorenal outcomes and reduced all-cause mortality, compared with DPP-4is [81]. Real-world data from the CVD-REAL Catalonia study (including 25,834 people with T2DM) demonstrated that SGLT2i use was associated with a lower risk of HF, all-cause mortality, modified MACE (all-cause mortality, myocardial infarction or stroke) and CKD compared with other glucose-lowering treatments (P < 0.001 for each outcome) [84].
Real-world models and examples that illustrate the value of newer T2DM treatments will become increasingly important as we move through the era of the COVID-19 pandemic, in which HCPs are facing additional financial constraints imposed by payers within healthcare systems. HCPs may therefore feel resistant to the prescribing of medicines that are perceived to be more expensive than traditional therapies. Rebuilding services after COVID-19 will require a huge investment of HCP time, money and resources. However, those able to take a long-term view will understand that an investment in SGLT2i therapies is likely to reduce the overall expenditure required for the downstream management of the CV and renal complications of T2DM and relieve some of the associated financial burden.
Rebuilding Type 2 Diabetes Care in the Era of COVID-19
Those involved in managing T2DM have met with many challenges during the COVID-19 pandemic due to cancelled face-to-face clinics and reduced access to health services across primary and secondary care. While HCPs continue to adapt to newer ways of interacting with patients under their care, the agile approach demonstrated by healthcare services and practitioners during the pandemic has seen services embrace the adoption of smarter approaches to care through the use of tools such as virtual technologies and telemedicine (e.g. telephone consultations).
The pandemic has had a varying impact on T2DM management, with outcomes influenced by local rates of infection and the ability of services to continue running during each of the lockdowns. Many people are likely to have ceased their T2DM medications due to periods of acute illness during the pandemic, and there may have been some hesitancy among HCPs in restarting treatments once individuals had recovered. It is important that people with diabetes re-establish their therapeutic regimen as soon as possible to ensure that they maintain good blood glucose control and manage their risk of complications. Studies are ongoing regarding the links between T2DM and the severity of COVID-19 disease, with some data suggesting that people with poorly controlled HbA1c may be at greater risk of adverse outcomes when hospitalised with COVID-19 infection, and elevated levels of systemic inflammation have been implicated in the underlying mechanisms of disease [85,86,87,88,89]. A reciprocal model has been proposed (Fig. 2) that links poor glycaemic control with associated inflammation, hypertension, CVD and other risk factors that increase the risk of progressive and severe COVID-19 infection [88]. Likewise, factors associated with severe COVID-19 disease may raise the risk of developing T2DM [88]. Biochemical stress due to infection could result in insulin resistance and insulin deficiency (caused by beta-cell damage), increasing the likelihood of T2DM onset [88]. This disease model emphasises the need for well-managed blood glucose in people with T2DM and for interventions that lower the incidence of risk factors, such as having overweight/obesity, which are linked to poor COVID-19 outcomes and adverse CV events [88].
Synopsis of the reciprocal effects of diabetes and COVID-19 [88]. The relationship between diabetes and COVID-19 is biunivocal. On one hand, people with diabetes have worse outcomes because of multiple associated conditions enhancing the risk. On the other hand, SARS-CoV-2, because of its tropism for the beta-cell, might cause new-onset diabetes or sustain hyperglycaemia at hospital admission. The impairment of beta-cell function along with the inflammatory cytokine storm and counter-regulatory hormonal responses can precipitate further acute metabolic complications (DKA or HHS). New-onset diabetes, hyperglycaemia at admission, and acute metabolic deterioration, in turn, can further worsen COVID-19 outcomes. DKA Diabetic ketoacidosis, HHS hyperglycaemic hyperosmolar syndrome, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2. Reproduced with permission from Apicella et al. [88]
Most people with T2DM are unlikely to experience severe COVID-19, but considerations should be in place for people with diabetes who contract the coronavirus. Better understanding is required of the pharmacological interventions with the potential to affect/improve outcomes for people with T2DM and COVID-19 as retrospective studies suggest that mortality may be reduced for individuals treated with metformin, SGLT2is and GLP-1 RAs [90]. In an RCT (the DARE-19 trial), dapagliflozin showed numerical reductions in CV and renal events for people with COVID-19 with/without T2DM, although the difference was not significant [90]. This is a developing area of research, and more RCTs are needed to understand this phenomenon fully. In the meantime, it is recommended that people with T2DM who experience periods of acute dehydrating illness (due to COVID-19 or other conditions) follow the sick day guidance outlined by the ABCD and Diabetes UK [44, 92,93,94,95,96]. The guidance recommends that relevant treatments (including SGLT2is) are ceased while the individual is unwell and should be restarted once the person is able to eat and drink normally [44, 92,93,94,95,96]. Practical recommendations for the management of T2DM in people with COVID-19 have been published by various groups, including an international panel of experts in the field of diabetes and endocrinology and the Spanish Endocrinology Society [97, 98]. Diabetes UK has also developed a position statement to support HCPs and people living with diabetes during the COVID-19 pandemic, which includes advice on delivery of remote healthcare services [99]. This is particularly relevant as 49% of people surveyed by Diabetes UK did not have contact with their HCP during the pandemic [99]. Uptake of vaccinations against COVID-19 should improve the prognosis for people with T2DM who catch the virus. However, issues within healthcare systems relating to suboptimal outpatient care are likely to persist for some time and it will be necessary to establish practical strategies to improve disease outcomes and provide durable expenditure reductions. Within such a scenario, proper prescribing of medicines that may ensure good glycaemic control and provide cardiorenal protection should be encouraged in the attempt to enhance cost-effectiveness of treatment of people with Type 2 diabetes.
Practicalities of Prescribing SGLT2i Treatments
The long-term management of T2DM and its associated complications/comorbidities represents a complex and challenging issue for clinicians, particularly in primary care where many practices are working to re-establish services after the initial impact of the coronavirus pandemic. HCPs with numerous competing priorities receive a continuous flow of prescribing and safety updates from international medicine regulators. In addition, local prescribing groups and committees will send regular updates on formulary decisions. There is pressure from payors to minimise disease management costs and HCPs must keep abreast of ongoing changes to clinical guidance from international and national bodies (e.g. International Diabetes Federation, NICE, Scottish Medicines Consortium) as well as relevant professional organisations (e.g. ABCD/UKKA, KDIGO, ADA, EASD). Although HCPs welcome data to support evidence-based prescribing, the volume of information emerging in the field of T2DM is vast and difficult to seamlessly apply in daily practice alongside existing clinical protocols.
As already discussed, the ever-expanding evidence base for SGLT2i treatments has resulted in changes to the licensed indications for some of the approved drugs in this class [59,60,61, 100]. The Improving Diabetes Steering Committee recommends that SGLT2i prescribing in T2DM should be implemented in accordance with the current ADA/EASD guidelines, which were developed in the context of the latest evidence [42]. The Steering Committee developed the ‘SGLT2i Prescribing Tool for T2DM Management’ in 2018 to support HCPs in the appropriate use of SGLT2is, and the Tool has been updated over subsequent years in line with the evolving evidence base. The latest version of the ‘SGLT2i Prescribing Tool for T2DM Management’ is provided in the Electronic Supplementary Material section of this paper and replaces those versions previously published [1,2,3]. In addition to the traffic light system, indicating the clinical situations in which SGLT2i therapy should be offered, considered or avoided for people with T2DM, a series of clinical summaries has been provided to give further explanation and support for prescribers. A brief overview of the situations included in the full Prescribing Tool are shown in Fig. 3. Prescribing of SGLT2i therapies must be within the licensed indication for each drug and the relevant summary of product characteristics (SmPC) should be consulted accordingly [59,60,61, 100]. The key themes covered in the ‘SGLT2i Prescribing Tool for T2DM Management’ include sick day guidance, practical advice on the management of DKA risk, use of SGLT2i therapies in people with T2DM and renal disease, drug–drug interactions (DDIs), genital and urinary infections, foot disease, Fournier’s gangrene, frailty and declining cognitive function, varying body mass index (BMI) and duration of T2DM, and dietary implications (e.g. low-calorie diets, eating disorders). These themes are also outlined below in further detail.
A brief guide to SGLT2i prescribing in T2DM [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36, 39,40,41,42,43,44, 59,60,61, 94, 95, 100]. The traffic light system indicates the appropriate approach for people with T2DM in each situation: green indicates when SGLT2i therapy should be offered; amber situations are when SGLT2i therapy can be considered; and SGLT2i therapy should not be prescribed for people within the red category. The full SGLT2i Prescribing Tool is provided in the Electronic Supplementary Material section. BMI body mass index, CKD chronic kidney disease, CV cardiovascular, CVD cardiovascular diseases, DKD diabetic kidney disease, eGFR estimated glomerular filtration rate, GLP-1 RA glucagon-like peptide 1 receptor agonist, LADA latent autoimmune diabetes in adults, PAD peripheral arterial disease, SGLT2i sodium glucose cotransporter-2 inhibitor, SmPC summary of product characteristics, T2DM Type 2 diabetes mellitus, UTIs urinary tract infections
People with Type 2 Diabetes Who are Most Likely to Benefit from SGLT2i Therapy
The ADA/EASD and ABCD/UKKA recommend the use of SGLT2is as second-line therapy to metformin (or first-line therapy in cases of intolerance to metformin) in adults with T2DM, while KDIGO guidelines recommend that people with T2DM and CKD are treated with metformin and an SGLT2i from the outset [42,43,44, 94]. Benefit may be gained from SGLT2i use among people with established CVD or high CV risk, CKD with albuminuria or high renal risk, a history of HF, inadequate glycaemic control with a need to minimise hypoglycaemia or inadequate glycaemic control with need to minimise weight gain/encourage weight loss [42,43,44, 94]. The decision to use an SGLT2i therapy with the aim of reducing MACE, HHF or progression of CKD should be made independently of baseline or target HbA1c [42,43,44, 94].
General Advice on SGLT2i Use During Periods of Acute Illness
Sick day guidance was developed to help people in managing their diabetes during intercurrent illness [92,93,94,95,96]. Throughout periods of acute illness (whether it is COVID-19 or any other illness), maintenance of glycaemic control can be challenging, and people with T2DM will be at increased risk of acute DKA and acute metabolic decompensation (i.e. hyperosmolar hyperglycaemic state) resulting from a reduced intake of food and liquids and the physiological mechanisms that come in to play while the body is fighting an infection (e.g. hormonal response, inflammation) [92, 95, 96]. DKA is discussed in further detail below in the context of SGLT2i treatments. People with T2DM should be advised about sick day guidance, be informed of how to recognise the signs and symptoms of DKA, have access to glucose and/or ketone testing (where appropriate) and understand when to seek assistance [92, 95, 96]. Individuals should aim to stay well hydrated and maintain their carbohydrate intake when they are unwell and should seek medical assistance if vomiting is persistent or they are unable to eat for a prolonged duration [92, 96].
The ‘SADMANS’ medication list (Box 1) has been widely adopted as an aid in identifying the medicines that should be suspended during intercurrent illness to reduce the risk of kidney function decline and other adverse events [90,91,92,93]. Treatment should be re-started within 24 h, once eating and drinking has returned to normal so that glycaemic control can be optimised once more.
Diabetic Ketoacidosis in People with T2DM
Although usually undetectable in blood (< 0.6 mmol/L) and urine (negative on urine dipsticks), ketone levels can be increased with SGLT2i treatments because energy metabolism is switched from carbohydrate to lipid utilisation [94, 99]. During periods of acute illness, stress hormone levels rise, causing increased risk of insulin resistance. When this occurs in combination with reduced endogenous insulin secretion, a state of relative insulin deficiency results that leads to free fatty acid breakdown and ketosis occurs [96, 101]. In a separate process, SGLT2is cause glycosuria and, therefore, continue to reduce plasma glucose concentration, which can lead to a state of normal glucose and raised ketones with acidosis, termed “euglycaemic DKA” [101]. Another theory suggests that rising sodium concentrations within the renal tubular fluid, due to reduced sodium reabsorption with SGLT2 inhibition, leads to an increased positive electrical charge within the tubular lumen and an influx of negatively charged ketone bodies. As a result, urinary ketone clearance will be reduced and plasma ketone levels will rise [101].
DKA is listed as a rare adverse event, affecting approximately 1 in 10,000 people with T2DM taking SGLT2i medications, as shown in CVOTs and renal studies for currently approved SGLT2is [5, 7, 11, 15, 22, 23, 102]. Low absolute incidence of DKA was reported in the canagliflozin CANVAS and CREDENCE studies, no imbalance was demonstrated for DKA episodes in the VERTIS CV trial (ertugliflozin), three episodes of DKA were reported in the dapagliflozin arms of the DAPA-HF study, two cases of ketoacidosis were reported in the placebo arm during the DAPA-CKD study and none were seen in either treatment arm for the EMPEROR–Reduced (empagliflozin) or DAPA-CKD (dapagliflozin) trials [5, 7, 11, 15, 22, 23, 102].
If DKA is suspected, HCPs should check ketone levels [96]. As recommended by the European Medicines Agency's (EMA)Pharmacovigilance Risk Assessment Committee (PRAC) and ABCD/UKKA, HCPs and people with T2DM should be aware of the key symptoms for DKA, which include in this setting nausea or vomiting, stomach pain, excessive thirst, fast and deep breathing, confusion, unusual sleepiness or tiredness, a sweet smell to the breath, a sweet/metallic taste in the mouth or a different odour to urine or sweat [44, 96, 102]. While ketone monitoring may be variable (or absent) across practices, some people with T2DM may be given dipsticks to allow them to check their ketone levels at home. If DKA is suspected, blood ketone testing would ideally be used to confirm (> 1.6 mmol/L) or exclude DKA [42, 96]. However, where blood ketone diagnostics are not readily available, HCPs and people with diabetes must be vigilant for the main signs of DKA so that urgent hospital admission can be implemented where DKA is suspected [96]. Groups that should not be treated with an SGLT2i due to DKA risk include those with T2DM with pancreatic dysfunction and Type 3c diabetes or previous DKA. For patients with Type 1 diabetes, only dapagliflozin 5 mg was previously approved, but has recently been withdrawn, due in part to ongoing concerns about risk of DKA [61]. Detailed discussion of SGLT2i use in Type 1 diabetes is beyond the scope of this review.
Monitoring of Kidney Function and Use of SGLT2i Therapies in People with Renal Dysfunction
The accumulation of robust data relating to the cardiorenal effects of SGLT2i treatments has resulted in the broadening of licensed indications for two of the currently available therapies, beyond the treatment of hyperglycaemia, to include people with reduced kidney function [59,60,61, 100]. Box 2 provides a summary of the current indications for people with impaired kidney function in the case of each approved SGLT2i therapy in Europe (N.B. the information provided is correct at the time of publication). The relevant product SmPC should be consulted before initiating therapy [59,60,61, 100]. Although SGLT2i treatments provide positive cardiorenal effects for people at high risk of HF and DKD, the glucose-lowering effect will be diminished in people with reduced kidney function due to their mechanism of action [59,60,61]. Local prescribing information should be reviewed to ensure that the appropriate dosage is given for people with T2DM and CKD [59,60,61]. Additional therapies may be required for people with T2DM and renal impairment in need of additional glycaemic control [59,60,61].

Monitoring of kidney function is usually recommended prior to initiating SGLT2i therapy and periodically during treatment [59,60,61, 100]. It is usual to see an initial acute reduction in estimated glomerular filtration rate (eGFR) at the start of SGLT2i treatment, which is typically followed by an overall slowing in the decline of eGFR over time and a reduction in other markers of kidney function deterioration [5, 26,27,28,29]. In most cases (> 98%), the dip in eGFR will be < 30% and the renal or CV prognosis will not worsen [5, 26,27,28, 103]. ABCD/UKKA recommend against early testing of kidney function following SGLT2i initiation on the basis that withdrawal of the therapy due to an initial decline in eGFR is inappropriate [44, 46].
The algorithm for DKD testing developed by Winocour et al. [105] (adapted in Fig. 4) was based on NICE and KDIGO recommendations and provides a useful reference regarding the appropriate frequency of eGFR and urine albumin to creatinine ratio (UACR) monitoring [43, 104, 105]. NICE and KDIGO recommend that baseline eGFR and UACR should be recorded and then eGFR monitored at least annually, or more frequently if eGFR falls below 30 mL/min/1.73 m2 [3, 43, 104, 105]. ADA guidelines recommend that patients with diabetes and urinary albumin > 30 mg/mmol creatinine and/or an eGFR of 30–60 mL/min/1.73 m2 should be monitored twice annually to guide therapy [42]. As eGFR and UACR are each independent risk factors for adverse outcomes and mortality, the Winocour et al. algorithm suggests that both should be monitored at least annually and more regularly in cases where UACR is > 30 mg/mmol (300 mg/g) and eGFR < 45 mL/min/1.73 m2 [105]. Acute kidney injury is not a concern for SGLT2i therapies [22, 27, 28, 32, 106].

Adapted from Winocour et al. [105] (https://diabetesonthenet.com/diabetes-primary-care/testing-for-kidney-disease-in-type-2-diabetes-consensus-statement-and-recommendations/)
Diagnostic monitoring for CKD/DKD in people with T2DM. a Algorithm for DKD testing, b frequency of monitoring per year by eGFR and adverse risk category for people with (or at risk of) CKD [43, 104, 105]. ABCD Association of British Clinical Diabetologists, AKI acute kidney injury, NICE National Institute for Health and Care Excellence, UACR urine albumin:creatinine ratio.
Drug–Drug Interactions and Use of Loop Diuretics Alongside SGLT2i Therapies
SGLT2i therapies can be prescribed alongside other commonly prescribed medications in T2DM management with no clinically relevant interactions expected [59,60,61, 100]. However, the label for each of the approved SGLT2i therapies advises caution and monitoring of volume status when prescribing in combination with diuretic medicines due to potential dehydrating and hypotensive effects [59,60,61, 100]. It is therefore important to check the individual SmPC before prescribing SGLT2i treatments alongside diuretics. People initiating SGLT2i therapy should be encouraged to drink plenty to maintain hydration and lower the risk of hypotension, with closer monitoring used for elderly individuals or those at risk of falls.
Large-scale RCTs and real-world studies examining SGLT2i medicines in people with T2DM and high CV or renal risk included people taking loop diuretics and thiazide diuretics [4,5,6, 27, 94, 107, 108]. In the CANVAS Program, individuals taking diuretic therapy at baseline showed a greater relative risk reduction for MACE with canagliflozin treatment compared with those not on baseline diuretics with no increase in renal or other adverse events [108]. Likewise, the CVD-REAL study showed that the rate of renal function decline was slowed with SGLT2i treatments, regardless of whether loop diuretics were co-administered [28].
The dosage of insulin or insulin secretagogues (e.g. SU therapies) should be initially lowered for individuals starting SGLT2i therapy to reduce the risk of hypoglycaemia [59,60,61, 100].
Genital and Urinary Infections
Genital mycotic infections and urinary tract infections (UTIs) may occur with SGLT2i treatment, particularly during the initial stages of treatment [94]. Women and individuals with previous genital mycotic infections are at higher risk of developing genital fungal infections with SGLT2i therapy [59,60,61, 100]. Genital infections following SGLT2i initiation are typically mild to moderate in nature and respond well to the conventional therapy, usually one single dose of oral fluconazole [94]. The appearance of genital candidiasis does not imply the withdrawal of SGLT2i treatment. Analysis of real-world data and meta-analysis of safety data from randomised trials did not suggest an increased risk of harm with SGLT2i inhibitors over placebo or active comparators with respect to UTIs [106, 109, 110]. Genital infections and UTIs should be treated with antifungals or antibiotics, respectively, with advice on hydration as well as hygiene given. SGLT2is should be stopped if infections are recurrent [44]. Urinary frequency/urgency may be increased with SGLT2i therapy [59,60,61, 100].
Fournier’s Gangrene
Fournier’s gangrene (necrotising fasciitis of the perineum) is a rare adverse event [111, 112]. In 2019, the UK Medicines and Healthcare products Regulatory Agency (MHRA) estimated that experience with SGLT2i treatment in the UK had accumulated to reach 548,565 patient-years, with just six yellow card reports relating to Fournier’s gangrene [44, 111]. Predisposing conditions for Fournier’s gangrene include diabetes and associated comorbidities, such as obesity, CKD and congestive HF [112]. Among the 17,160 people included in the DECLARE-TIMI 58 trial, five incidents of Fournier’s gangrene were reported in the placebo group and one in the dapagliflozin group [6]. One case was reported in the empagliflozin arm of the EMPEROR-reduced study, which included 5988 participants [10, 113]. Patients treated with SGLT2is should be made aware of this very rare but serious infection and advised to seek urgent medical attention if they experience severe pain, tenderness, worsening redness or swelling in the genital or perineal area [44, 111].
Foot Disease and Lower Limb Amputations
Among the numerous RCTs and real-world studies examining the efficacy and safety of SGLT2i therapies, excess amputations were reported in one randomised trial and low incidence has been shown across all published RCTs (0–2 additional events per 1000 person-years) [4,5,6, 22]. Current ADA/EASD guidelines recommend that the relative risks should be discussed fully with people who have active foot disease or those at elevated risk for amputation, with the decision to prescribe an SGLT2i therapy being shared between the clinician and individual with T2DM [42]. People with T2DM, including those treated with SGLT2is, must be educated regarding preventative foot care, and comprehensive checks should be conducted regularly. The individual must contact their HCP promptly in cases where wounds, discoloration or pain are identified and therapy ceased if significant problems occur (e.g. infection) [42, 44, 94].
Frailty and Declining Cognitive Function
SGLT2i treatments should be used with caution in people who are living with moderate or severe frailty because of increased risk of weight loss favouring sarcopenia, genital candidiasis and UTIs, urinary incontinence, fluid volume depletion and dehydration [114]. Volume depletion can cause hypotension and postural dizziness that increase the chances of experiencing falls and bone fractures [114]. Blood pressure monitoring should be conducted, particularly for those at risk of falls and for people taking diuretic medicines [43]. Advice should be given to the individual and those who care for them regarding the importance of remaining well-hydrated and reporting any symptoms of dizziness [44, 114]. Research using animal models suggests that SGLT2i therapies may slow the development of Alzheimer’s disease; however, further studies are required to fully understand the effects of these treatments in this setting and their influence on the mechanisms of disease [115, 116].
Cases of High Blood Glucose Despite Oral Diabetes Medication
In cases where blood glucose remains high, despite therapy, the person’s clinical history should be checked to establish whether an underlying undiagnosed condition that is also associated with hyperglycaemia is likely (e.g. inflammatory disease) and requirement for insulin replacement therapy should be evaluated. Assessment of BMI should be conducted and any signs of recent weight loss noted. Individuals may also require assessment for latent autoimmune diabetes in adults (LADA) via testing for antibodies against glutamic acid decarboxylase antibodies (GADA) and possibly islet antigen 2 (IA2) and zinc transporter 8 (ZnT8) [117].
People with T2DM who have progressed to the stage of requiring insulin therapy and/or insulin secretagogues may have to reduce the dosage of these medicines when initiating SGLT2i therapy to reduce the risk of hypoglycaemia and should be guided by their HCP [44, 94]. If insulin requirement reduces significantly over time with SGLT2i therapy, monitoring for DKA should be implemented (testing for urinary and/or blood ketones, where diagnostics are available) [42].
Dietary Implications
Individuals with T2DM taking SGLT2i medication who restrict their food or fluid intake for any reason will risk becoming severely dehydrated and being at greater risk of developing ketoacidosis [42]. When an individual is unable to eat or drink regularly due to illness, they should cease their SGLT2i medication until they are able to take food and fluids normally again [42, 92, 95]. People on low carbohydrate, very low calorie or ketogenic diets should not be selected for SGLT2i treatment due to their increased risk of developing ketoacidosis [44]. Similarly, SGLT2i therapy is not recommended for people living with T2DM and a co-morbid eating disorder (e.g. anorexia nervosa) or elderly people with unreliable eating patterns. Individuals with T2DM who choose to follow an intermittent fasting diet (e.g. 5:2 diet) must have their diabetes regimen reviewed, with therapies such as SGLT2is stopped or their dosage reduced on fasting days. As highlighted above, people with T2DM must be educated regarding the key signs and symptoms of DKA and understand when they should seek urgent medical advice/assistance [44, 96].
Conclusion
As the prevalence of T2DM continues to increase alongside the enduring global obesity epidemic, HCPs are faced with increasing numbers of people who have associated CV and renal risk factors or established disease. Interruptions to the provision and continuity of care caused by the ongoing COVID-19 pandemic have increased pressure on already overstretched services. As a result, access to effective treatment options that help to avoid serious, costly and debilitating cardiorenal events are a higher priority than ever. The abundance of evidence supporting the protective cardiorenal effects of SGLT2i therapies has driven a shift in perceptions among the clinical community regarding their value in treating high-risk T2DM populations, and the newly revised therapeutic indications for the available SGLT2is reflect their efficacy within these settings. By addressing the practicalities of SGLT2i prescribing in clinical practice in this article and through the provision of the newly created the ‘SGLT2i Prescribing Tool for T2DM Management’, the Improving Diabetes Steering Committee hopes to support HCPs in advancing their understanding regarding the appropriate place of SGLT2i treatments within the T2DM treatment pathway with the aim of improving patient care.
References
Wilding J, Fernando K, Milne N, et al. SGLT2 inhibitors in Type 2 diabetes management: key evidence and implications for clinical practice. Diabetes Ther. 2018;9(5):1757–73.
Ali A, Bain S, Hicks D, Newland, et al. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c-translating evidence into practice. Diabetes Ther. 2019;10(5):1595–622.
Wheeler DC, James J, Patel D, et al. SGLT2 inhibitors: slowing of chronic kidney disease progression in Type 2 diabetes. Diabetes Ther. 2020;11(12):2757–74.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in Type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
Cosentino F, Cannon CP, Cherney DZI, et al. Efficacy of ertugliflozin on heart failure-related events in patients with Type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142(23):2205–15.
Packer M, Butler J, Zannad F, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and a preserved ejection fraction: the EMPEROR-Preserved trial. Circulation. 2021. 144(16):1284–94
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143(4):326–36.
Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation. 2021;143(4):337–49.
Silva-Cardoso J, Andrade A, Brito D, et al. SGLT-2 inhibitors: a step forward in the treatment of heart failure with reduced ejection fraction. Rev Port Cardiol (Engl Ed). 2021;S0870–2551(21):00149–59.
Savarese G, Uijl A, Lund LH, et al. Empagliflozin in heart failure with predicted preserved versus reduced ejection fraction: data from the EMPA-REG OUTCOME trial. J Card Fail. 2021;27(8):888–95.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249–59.
Khunti K, Kosiborod M, Kim DJ, et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors vs other glucose-lowering drugs in 13 countries across three continents: analysis of CVD-REAL data. Cardiovasc Diabetol. 2021;20(1):159.
Lam CSP, Karasik A, Melzer-Cohen C, et al. Association of sodium-glucose cotransporter-2 inhibitors with outcomes in type 2 diabetes with reduced and preserved left ventricular ejection fraction: Analysis from the CVD-REAL 2 study. Diabetes Obes Metab. 2021;23(6):1431–5.
Kohsaka S, Lam CSP, Kim DJ, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606–15.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;38:2295–306.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Cherney DZI, Charbonnel B, Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–67.
Lo KB, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10:1–10.
Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35.
Pasternak B, Wintzell V, Melbye M, et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: scandinavian cohort study. BMJ. 2020;369:m1186.
Filion KB, Lix LM, Yu OH, et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi-database retrospective cohort study. BMJ. 2020;23(370):m3342.
Cherney DZI, Dagogo-Jack S, McGuire DK, et al. Kidney outcomes using a sustained ≥40% decline in eGFR: a meta-analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021. 44(8):1139–43.
Neuen BL, Ohkuma T, Neal B, et al. Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: findings from the CANVAS Program. Am J Kidney Dis. 2021;77(1):23–34.
Verma S, Leiter LA, Zinman B, et al. Time to cardiovascular benefits of empagliflozin: a post hoc observation from the EMPA-REG OUTCOME trial. ESC Heart Fail. 2021;8(4):2603–7.
Yanai H, Hakoshima M, Adachi H, Katsuyama H. Multi-organ protective effects of sodium glucose cotransporter 2 inhibitors. Int J Mol Sci. 2021;22(9):4416.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19(1):98.
Xu J, Hirai T, Koya D, Kitada M. Effects of SGLT2 inhibitors on atherosclerosis: lessons from cardiovascular clinical outcomes in type 2 diabetic patients and basic researches. J Clin Med. 2021;11(1):137.
Cappetta D, De Angelis A, Bellocchio G, et al. Sodium-glucose cotransporter 2 inhibitors and heart failure: a bedside-to-bench journey. Front Cardiovasc Med. 2021;8: 810791.
Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–76.
Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):185.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–8.
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–115.
Dashora U, Gregory R, Winocour P, et al. Association of British Clinical Diabetologists (ABCD) and Diabetes UK joint position statement and recommendations for non-diabetes specialists on the use of sodium glucose co-transporter 2 inhibitors in people with type 2 diabetes (January 2021). Clin Med (Lond). 2021;21(3):204–10.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
Tuttle KR, Brosius FC 3rd, Cavender MA, et al. SGLT2 inhibition for CKD and cardiovascular disease in Type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Diabetes. 2021;70(1):1–16.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2015 (updated 24 November 2021). https://www.nice.org.uk/guidance/NG28. Accessed Jan 2022.
Arnold SV, Tang F, Cooper A, et al. Global use of SGLT2 inhibitors and GLP-1 receptor agonists in Type 2 diabetes: results from DISCOVER. Diabetes. 2021;70(Suppl 1):324-OR.
Eberly LA, Yang L, Eneanya ND, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4): e216139.
Michelli A, Cimino E, Manicardi V, et al. Annali AMD 2020—Sinossi sul Diabete Tipo 2 Valutazione degli indicatori AMD di qualità dell’assistenza al diabete di tipo 2 in Italia. JAMD. 2021;24:19–29 (in Italian).
Fadini GP, Tentolouris N, Caballero Mateos I, Bellido Castañeda V, Morales PC. A multinational real-world study on the clinical characteristics of patients with Type 2 diabetes initiating dapagliflozin in Southern Europe. Diabetes Ther. 2020;11(2):423–36.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37.
Mata-Cases M, Franch-Nadal J, Real J, Vlacho B, Gómez-García A, Mauricio D. Evaluation of clinical and antidiabetic treatment characteristics of different sub-groups of patients with type 2 diabetes: data from a Mediterranean population database. Prim Care Diabetes. 2021;15(3):588–95.
Khunti K, Charbonnel B, Cooper A, et al. Associations between second-line glucose-lowering combination therapies with metformin and HbA1c, body weight, quality of life, hypoglycaemic events and glucose-lowering treatment intensification: the DISCOVER study. Diabetes Obes Metab. 2021;23(8):1823–33.
Moreno Juste A, Menditto E, Orlando V, et al. Treatment patterns of diabetes in Italy: a population-based study. Front Pharmacol. 2019;6(10):870.
Société Francophone du Diabéte. la dapagliflozine (FORXIGA®, XIGDUO®) est disponible en France pour le traitement du diabète de type 2 de l’adulte. 2020. https://www.sfdiabete.org/mediatheque/kiosque/editoriaux/avril-2020-la-dapagliflozine-forxigar-xigduor-est-disponible-en. Accessed Jan 2022.
Scheen AJ. À propos de l’expérience belge avec les inhibiteurs des SGLT2. About the Belgian experience with SGLT2 inhibitors. Médecine Des Maladies Métaboliques 2020;14(4):320–30 (in French)
National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes technology appraisal guidance (TA390). 2016. https://www.nice.org.uk/guidance/ta390. Accessed Jan 2022
Napp Pharmaceuticals Limited. Canagliflozin summary of product characteristics. December 2021. https://www.medicines.org.uk/emc/product/8855/smpc. Accessed Jan 2022
Boehringer Ingelheim Limited. Empagliflozin summary of product characteristics. December 2021. https://www.medicines.org.uk/emc/product/7703/smpc. Accessed Jan 2022
AstraZeneca UK Limited. Dapagliflozin summary of product characteristics. November 2021. https://www.medicines.org.uk/emc/product/7607/smpc. Accessed Jan 2022
Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–62.
Whicher CA, O’Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020;37(2):242–7.
Jodar E, Artola S, Garcia-Moll X, et al. Incidence and costs of cardiovascular events in Spanish patients with type 2 diabetes mellitus: a comparison with general population, 2015. BMJ Open Diabetes Res Care. 2020;8(1):e001130.
Mata-Cases M, Casajuana M, Franch-Nadal J, et al. Direct medical costs attributable to type 2 diabetes mellitus: a population-based study in Catalonia. Spain Eur J Health Econ. 2016;17(8):1001–10.
Ulrich S, Holle R, Wacker M, et al. Cost burden of type 2 diabetes in Germany: results from the population-based KORA studies. BMJ Open. 2016;6(11): e012527.
Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90–9.
Nakajima H, Okada S, Mohri T, et al. Dapagliflozin improves treatment satisfaction in overweight patients with type 2 diabetes mellitus: a patient reported outcome study (PRO study). Diabetol Metab Syndr. 2018;1(10):11.
Newman TV, Munshi KD, Neilson LM, et al. Health care utilization and costs associated with switching from DPP-4i to GLP-1RA or SGLT2i: an observational cohort study. J Manag Care Spec Pharm. 2021;27(4):435–43.
Ehlers LH, Lamotte M, Monteiro S, et al. The cost-effectiveness of empagliflozin versus liraglutide treatment in people with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2021;12(5):1523–34.
Willis M, Nilsson A, Kellerborg K, et al. Cost-Effectiveness of canagliflozin added to standard of care for treating diabetic kidney disease (DKD) in patients with Type 2 diabetes mellitus (T2DM) in England: estimates using the CREDEM-DKD model. Diabetes Ther. 2021;12(1):313–28.
Taylor RS, Sadler S, Dalal HM, et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: A decision model-based analysis. Eur J Prev Cardiol. 2019;26(12):1252–61.
Najafzadeh M, Pawar A, Déruaz-Luyet A, et al. PDB128 Reduced healthcare utilization in patients using empagliflozin: an interim analysis from the empagliflozin comparative effectiveness and safety (EMPRISE) study. Value Health. 2019;22:S161.
Pawar A, Patorno E, Déruaz-Luyet A, et al. PDB126 Comparative healthcare costs and medication burden in real-world patients augmenting metformin monotherapy with empagliflozin from the empagliflozin comparative effectiveness and safety (EMPRISE) study. Value Health. 2019;22:S161.
Manceur AM, Durkin M, Kharat A, et al. Costs associated with renal and cardiovascular events among patients with type 2 diabetes mellitus and nephropathy: a cost model based on the CREDENCE clinical trial. Curr Med Res Opin. 2020;36(4):563–70.
McEwan P, Morgan AR, Boyce R, et al. The cost-effectiveness of dapagliflozin in treating high-risk patients with type 2 diabetes mellitus: an economic evaluation using data from the DECLARE-TIMI 58 trial. Diabetes Obes Metab. 2021;23(4):1020–9.
Usó-Talamantes R, González-de-Julián S, Díaz-Carnicero J, et al. Cost of Type 2 diabetes patients with chronic kidney disease based on real-world data: an observational population-based study in Spain. Int J Environ Res Public Health. 2021;18(18):9853.
Russo G, Monami M, Perseghin G, Avogaro A, et al. The “early treatment” approach reducing cardiovascular risk in patients with Type 2 diabetes: a consensus from an expert panel using the Delphi technique. Diabetes Ther. 2021;12(5):1445–61.
Mannucci E, Mangia PP, Pradelli L. Clinical and economic rationale for the early use of SGLT2 inhibitors in patients with Type 2 diabetes. Farmeconomia Health Econom and Therap Path. 2020;21(Suppl 1):3–20.
Milne N, Di Rosa F, Findlow L, Arris M, Rutter MK, Kanumilli N. Late to the party: Two-year outcomes of the Manchester CoDES (Community Diabetes Education and Support) pilot. J Diabetes Nurs. 2021;25:03.
Fadini GP, Solini A, Manca ML, et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(2):252–60.
Morieri ML, Consoli A, Sesti G, et al. Comparative effectiveness of dapagliflozin vs DPP-4 inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes Metab Res Rev. 2021;37(1): e3353.
Longato E, Bonora BM, Di Camillo B, et al. Outcomes of patients with type 2 diabetes treated with SGLT-2 inhibitors versus DPP-4 inhibitors. An Italian real-world study in the context of other observational studies. Diabetes Res Clin Pract. 2021;179:109024.
Real J, Vlacho B, Ortega E, et al. Cardiovascular and mortality benefits of sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus: CVD-Real Catalonia. Cardiovasc Diabetol. 2021;20(1):139.
Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168: 108374.
Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23(3):870–4.
Coppelli A, Giannarelli R, Aragona M, et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–8.
Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–92.
Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing Type 2 diabetes. Cell Metab. 2020;31(6):1068–77.e3.
Khunti K, Knighton P, Zaccardi F, et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9(5):293–303.
Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(9):586-94
Diabetes UK. Dealing with illness. https://www.diabetes.org.uk/guide-to-diabetes/life-with-diabetes/illness. Accessed Jan 2022
Diabetes Canada. Sick-day medication list. Can J Diabetes. 2018;42(Suppl 1):S316.0.
Brown P. How to use SGLT2 inhibitors safely and effectively. Diabetes Prim Care. 2021;23(1):5–7.
Down S. How to advise on sick day rules. Diabetes Prim Care. 2020;22:47–8.
Diggle J. Ketones and diabetes. Diabetes Prim Care. 2020;22:49–50.
Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–50.
Sociedad Española de Endocrinología Nutrición (SEEN). Recomendaciones coronavirus (COVID-19) y personas con diabetes. https://facme.es/wp-content/uploads/2020/03/recomendaciones-DM_COVID-19_SEENp-1.pdf. (In Spanish). Accessed Jan 2022.
Diabetes UK. Diabetes care during the COVID-19 pandemic. Position statement. December 2020. https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/public/2020-12/Position%20statement%20on%20diabetes%20care%20during%20the%20Covid-19%20pandemic.pdf. Accessed Jan 2022
Merck Sharp & Dohme (UK) Limited. Ertugliflozin summary of product characteristics. March 2021. https://www.medicines.org.uk/emc/product/10099/smpc. Accessed Jan 2022.
Rajeev SP, Wilding JPH. SGLT2 inhibition and ketoacidosis—should we be concerned? Brit J Diabetes Vasc Medicine. 2015;15(4):155–8.
European Medicines Association. SGLT2 inhibitors: PRAC makes recommendations to minimise risk of diabetic ketoacidosis. 2016. https://www.ema.europa.eu/en/news/sglt2-inhibitors-prac-makes-recommendations-minimise-risk-diabetic-ketoacidosis. Accessed Jan 2022.
Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99(3):750–62.
National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. NICE guideline ng203. 2021. https://www.nice.org.uk/guidance/ng203/resources/chronic-kidney-disease-assessment-and-management-pdf-66143713055173. Accessed Jan 2022
Winocour PH, Diggle J, Davies S, et al. Testing for kidney disease in type 2 diabetes: consensus statement and recommendations. Diabetes Primary Care. 2020;22:99–109.
Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9: e022577.
Pellicori P, Fitchett D, Kosiborod MN, et al. Use of diuretics and outcomes in patients with type 2 diabetes: findings from the EMPA-REG OUTCOME trial. Eur J Heart Fail. 2021;23(7):1085–93.
Yu J, Arnott C, Neuen BL, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS Program. ESC Heart Fail. 2021;8(2):1482–93.
Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium-glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: a population-based cohort study. Ann Intern Med. 2019;171(4):248–56.
Varshney N, Billups SJ, Saseen JJ, Fixen CW. Sodium-glucose cotransporter-2 inhibitors and risk for genitourinary infections in older adults with type 2 diabetes. Ther Adv Drug Saf. 2021;29(12):2042098621997703.
Medicines and Healthcare products Regulatory Agency. SGLT2 inhibitors: reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum). 2019. https://www.gov.uk/drug-safety-update/sglt2-inhibitors-reports-of-fournier-s-gangrene-necrotising-fasciitis-of-the-genitalia-or-perineum. Accessed Jan 2022
Sparenborg JD, Brems JA, Wood AM, Hwang JJ, Venkatesan K. Fournier’s gangrene: a modern analysis of predictors of outcomes. Transl Androl Urol. 2019;8(4):374–8.
Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29.
Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Ther. 2021;12(5):1227–47.
Hierro-Bujalance C, Infante-Garcia C, Del Marco A, et al. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res Ther. 2020;12(1):40.
Esterline R, Oscarsson J, Burns J. A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer’s disease? Int Rev Neurobiol. 2020;155:113–40.
Diabetes UK. Latent autoimmune diabetes in adults (LADA). https://www.diabetes.org.uk/diabetes-the-basics/other-types-of-diabetes/latent-autoimmune-diabetes. Accessed Jan 2022
Acknowledgements
Funding
The Improving Diabetes Steering Committee is supported by an educational grant from Napp Pharmaceuticals Limited and Mundipharma Research Limited. Napp has reviewed this document for factual accuracy only. Sponsorship and the journal’s Rapid Service Fee for this study were funded by Mundipharma Research Limited.
Medical Writing Assistance
Medical writing services were provided on behalf of the Steering Committee by Rebecca Down at Copperfox Communications Limited. Support for this assistance was funded by Mundipharma Research Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author Contributions
John PH Wilding, Marc Evans, Kevin Fernando, Jose Luis Gorriz, Ana Cebrian, Jane Diggle, Debbie Hicks, June James, Philip Newland-Jones, Amar Ali, Stephen Bain, Andrea Da Porto, Dipesh Patel, Adie Viljoen, David C Wheeler and Stefano Del Prato devised and shaped the content for the narrative review paper. Each of the named authors reviewed and critically appraised the manuscript during development and approved the final version for publication.
Disclosures
John PH Wilding undertakes consultancy and speaking engagements for industry contracted via the University of Liverpool (no personal payment) in relation to obesity and Type 2 diabetes; in the last 2 years this includes work for Alnylam, AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Napp, Novo Nordisk, Mundipharma, Pfizer, Rhythm Pharmaceuticals, Saniona and Ysopia. He is a named grant holder (at University of Liverpool) for research grants for clinical trials from AstraZeneca and Novo Nordisk, and he has has received honoraria/lecture fees from AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Mundipharma, Sanofi and Takeda. Marc Evans has received research support from Novo Nordisk and Takeda; and received speaker fees and honoraria from Boehringer Ingelheim, Janssen, MSD, Napp Pharmaceuticals, Novartis and Novo Nordisk. He is also the Editor-in-Chief of Diabetes Therapy. Kevin Fernando has received speaker honoraria from all current manufacturers of SGLT2 inhibitors: AstraZeneca, Janssen, Boehringer Ingelheim, Napp Pharmaceuticals and Merck. Jose Luis Gorriz has received funding for attending advisory boards from AstraZeneca, Janssen, Mundipharma, Boehringer Ingelheim, MSD, Novo Nordisk and Novartis; received institutional research grant support from AstraZeneca; received funding for clinical trials from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk and Bayer; and received speaker fees and honoraria from Boehringer Ingelheim, Lilly, Mundipharma, AstraZeneca, Novartis, Astellas and Novo Nordisk. Ana Cebrian has received research support from Merck Sharpe and Dohme and speaker honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck Sharpe and Dohme, Mundipharma, Novartis Pharmaceuticals, Novo Nordisk and Sanofi; and also received funding for attending advisory boards from AstraZeneca, Janssen, Mundipharma, Boehringer, Ingelheim MSD, Novo Nordisk, Novartis and Sanofi. Jane Diggle has received funding from the following companies for provision of educational sessions and documents and for attending advisory boards: Abbott, BD, B Braun, Boehringer Ingelheim, BMS, AstraZeneca, Eli Lilly, GlucoRx, Janssen, MSD, Napp Pharmaceuticals, Novo Nordisk, Owen Mumford, Sanofi and Takeda. June James has received funding for provision of educational sessions, resources and attendance at advisory boards from AstraZeneca, BD, Boehringer Ingelheim, Janssen, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk and Sanofi, Abbott, Mylan, Neon Diagnostics and Nipro; she does not hold any shares in any pharmaceutical company. Philip Newland-Jones has received sponsorship for education events, undertaken research for, or have received fees for educational speaking from, Abbott, AstraZeneca, Boehringer Ingelheim, Sanofi, Eli Lilly, Janssen, Novo Nordisk, Animas, LifeScan, MSD, Mylan, Napp, Servier and Takeda. Amar Ali has received speaker honoraria from all current manufacturers of SGLT2 inhibitors: AstraZeneca, Janssen, Boehringer Ingelheim, Napp Pharmaceuticals and Merck. Stephen Bain has received research support from Healthcare and Research Wales (Welsh Government) and honoraria from Boehringer Ingelheim, Lilly, Merck, Napp Pharmaceuticals, Novo Nordisk and Sanofi. He has ownership interest in Glycosmedia (diabetes online news service). He is also a member of the Editorial Board of Diabetes Therapy. Andrea Da Porto has received funding for attending advisory boards from Mundipharma, Boehringer Ingelheim and NovoNordisk; and has received speaker fees and honoraria from Boehringer Ingelheim, Lilly, Mundipharma, AstraZeneca and Novo Nordisk, Sanofi-Aventis and Takeda. Debbie Hicks has received speaker and advisory board honoraria and educational sponsorship from Abbott, AstraZeneca, B. Braun, BD, Boehringer Ingelheim, Gendius, GlucoRx, Janssen, Lifescan, Lilly, MSD, Mylan, Napp Pharmaceuticals, Novo Nordisk, Owen Mumford Roche, Sanofi and Takeda; she does not hold any shares in any pharmaceutical company. Dipesh Patel has received educational sponsorship, lecture fees and/or advisory related honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Napp Pharmaceuticals, Novo Nordisk, Sanofi, MSD and Takeda. Adie Viljoen has conducted research studies funded by, served as advisor to and received lecture honoraria and travel support from Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk, Pfizer, Regeneron, Sanofi Aventis and Tosoh. David C Wheeler has received honoraria, consultancy fees, lecture fees and/or travel support from Amgen, AstraZeneca, Astellas, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Lilly, Napp Pharmaceuticals, Mundipharma, Merck Sharp and Dohme, Takeda, Vifor and Zydus. Stefano Del Prato has received research support from AstraZeneca and Boehringer Ingelheim and funding for provision of educational sessions, resources and attendance at advisory boards from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharpe and Dohme, Novartis Pharmaceuticals, Novo Nordisk and Sanofi.
Compliance with Ethics Guidelines
This article is based upon previously conducted studies and does not involve any new studies of human or animal subjects. Some authors were involved in studies discussed within the paper that included human subjects, all of which complied with the tenets of the Declaration of Helsinki of 1964 and subsequent revisions and used protocols that had been approved by relevant Institutional Review Boards/Ethics Committees and included patients who had provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the Electronic Supplementary Material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wilding, J.P.H., Evans, M., Fernando, K. et al. The Place and Value of Sodium-Glucose Cotransporter 2 Inhibitors in the Evolving Treatment Paradigm for Type 2 Diabetes Mellitus: A Narrative Review. Diabetes Ther 13, 847–872 (2022). https://doi.org/10.1007/s13300-022-01228-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01228-w