Abstract
Introduction
International and Danish guidelines recommend the use of glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium–glucose cotransporter 2 (SGLT-2) inhibitors already in second line in the management of type 2 diabetes (T2D). The objective of this study was to evaluate the long-term cost-effectiveness (CE) of subcutaneous (SC) semaglutide (GLP-1 RA) versus empagliflozin (SGLT-2 inhibitor) in individuals with T2D uncontrolled on metformin alone from a Danish payer’s perspective.
Methods
Cost-effectiveness analyses (CEA) were conducted from a Danish payer’s perspective, using the IQVIA Core Diabetes model (CDM 9.5), with a time horizon of 50 years and an annual discount of 4% on costs and effects. Patients received either SC semaglutide or empagliflozin, in addition to metformin, until HbA1c threshold of 7.5% (58 mmol/mol) was reached, following which treatment intensification with insulin glargine in addition to empagliflozin or SC semaglutide plus metformin was considered. Baseline cohort characteristics and treatment effects were sourced from a published CEA. Utilities and cost of diabetes-related complications were also obtained from published sources. Treatment costs were derived from Danish official sources. Scenario analyses were also performed to test the accuracy of the base case results.
Results
Individuals with T2D on SC semaglutide plus metformin gained 0.065 life-years (LYs) and 0.130 quality-adjusted LYs (QALYs), respectively, at an incremental cost of DKK 96,923 (€ 13,031) compared to empagliflozin plus metformin, resulting in an incremental cost-effectiveness ratio (ICER) of DKK 745,561(€ 100,239) per QALY gained. The probabilistic sensitivity analysis (PSA) results showed that the SC semaglutide plus metformin was cost-effective in 19% of simulations assuming a willingness-to-pay (WTP) threshold of DKK 357,100 (€ 48,011)/QALY gained. Duration of therapy with SC semaglutide seems the key driver of results.
Conclusion
The current analyses suggest that SC semaglutide plus metformin is not cost-effective compared to empagliflozin plus metformin from a Danish payer’s perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The cost of management of diabetes is significant, and most of these costs are driven by individuals with major complications such as CVD and heart failure |
In line with international guidelines, Danish clinical guidelines recommend metformin followed by either SGLT-2 inhibitors or subcutaneous injectable medications for the treatment of T2D |
As treatment with injectable GLP-1 receptor agonists or oral delivery of SGLT-2 inhibitors, in addition to metformin, is to be continued long term, it is important to understand the cost-effectiveness of these therapies in the management of T2D |
An analysis of the long-term cost-effectiveness of treatment with SC semaglutide added to metformin compared to the most used SGLT-2 inhibitor, empagliflozin added to metformin, in people with T2D with inadequate glycaemic control on metformin alone was conducted from a Danish payer perspective |
IQVIA CDM version 9.5 model was used to predict the long-term clinical and economic results based on literature findings, clinical trials, and certain assumptions |
What was learned from the study? |
SC semaglutide plus metformin does not seem to be cost-effective versus empagliflozin plus metformin for the treatment of individuals with T2D with inadequate glycaemic control on metformin alone in the Danish setting |
Results of these cost-effectiveness analyses (CEA) were driven mainly by treatment duration, reflecting the unit cost difference between SC semaglutide and empagliflozin |
Introduction
According to the International Diabetes Federation (IDF), worldwide 463 million adults were living with diabetes in 2019, and type 2 diabetes (T2D) accounted for almost 90% of all the diabetes cases [1]. More than 240,000 Danes had T2D in 2020, which is expected to increase to 430,000 by 2030 [2, 3]. It is further predicted that the proportion of individuals with T2D will increase from 43 to 46% for women and from 38 to 45% for men in elderly populations (≥ 70 years) in Denmark [4]. In 2020, the cost of diabetes medication was the largest component on the primary care drug budget in Denmark, primarily owing to the rapidly increasing Danish population with T2D [5, 6].
T2D leads to inadequate glycaemic control, which is further associated with the development of macrovascular complications (cardiovascular diseases [CVD], stroke, myocardial infarction [MI] and angina pectoris) and microvascular complications (retinopathy, nephropathy and neuropathy) [7,8,9]. This was supported by the results from a previous database survey in primary care in Denmark which showed high prevalence (21.4%) of CVD in individuals with T2D [10]. Evidence suggests that achieving an early and intensive glycaemic control can reduce diabetic-related complications and lead to substantial savings for the Danish society [5, 11].
Glycaemic control can be achieved, according to individualised glycated haemoglobin A1C (HbA1c) targets, initially using metformin together with lifestyle interventions [7]. Timely intensification of the treatment of chronic, progressive conditions such as T2D is important to maintain glycaemic control and decrease the risk of diabetes-related complications and long-term healthcare costs [7]. When glycaemic control is no longer achieved with metformin alone, dual therapy with glucose-lowering agents (GLP-1 receptor agonists or SGLT-2 inhibitors) is recommended, considering the clinical characteristics of the patient, according to the consensus guidelines of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [7, 12,13,14,15]. In line with these international guidelines, Danish clinical guidelines also recommend lifestyle changes and advocate a stepwise approach to achieve glycaemic control that initiates with metformin, followed by treatment intensification with dual and triple therapy if HbA1c is at a certain level (7.5–9.0% and > 9.0%, respectively) [16, 17].
The clinical guidelines, both international and in Denmark, are based on the 2008 US Food and Drug Administration (US FDA) recommendation and subsequent European Medicines Agency (EMA) requirement that all glucose-lowering drugs should demonstrate a rigorous cardiovascular safety profile [5, 13, 18,19,20,21]. Injectable solution of semaglutide with a dose of 1 mg once weekly can be added to existing treatment (i.e. metformin) to improve diabetes-related effect measurement and outcomes in individuals with T2D [22]. Empagliflozin is also recommended at a dose of 10 mg and 25 mg for the treatment of individuals with T2D with and without CVD, heart failure and/or renal disease with an expected improvement in cardiac, renal, and metabolic outcomes. [23,24,25].
The Danish Medicines Agency has a history of promoting rational use of pharmaceuticals, interpreted as treatments that have the greatest effect, the fewest and least serious side effects, and the lowest price [26,27,28]. Thus, the recent clinical guidelines from the Danish Health Authorities recommend SGLT-2 inhibitors over GLP-1 receptor agonists in individuals with T2D because they have similar effect and safety profiles, but prices of GLP-1 receptor agonists are remarkably higher in Denmark [2].
From a health economics perspective, however, drug prices should not be the only economic input for the definition of rational drug use [29]. The price of the pharmaceutical is but one aspect of the total costs of the alternative treatments, and a full health economic evaluation with a long-term horizon should be conducted before such national recommendations can be made. It may be the case, for instance, that long-term savings in public healthcare offsets the extra short-term budget expenses of innovative drugs.
Direct clinical comparisons between an SGLT-2 inhibitor and a GLP-1 receptor agonist are very scarce. Recently, Capehorn et al. [30] used pooled data from clinical trials to evaluate the cost-effectiveness of subcutaneous (SC) semaglutide versus empagliflozin in the UK setting. The current study utilized the cohort characteristics and clinical data presented by Capehorn et al. [30] to assess the long-term cost-effectiveness of SC semaglutide plus metformin versus empagliflozin plus metformin in Danish settings using the IQVIA Core Diabetes model (CDM version 9.5). This approach considers individuals with T2D who have inadequate glycaemic control on metformin monotherapy.
Methods
Modelling Approach
Using the IQVIA CDM version 9.5, long-term projections of clinical and cost outcomes were performed from the Danish payer’s perspective. CDM is a proprietary, interactive, internet-based computer simulation model developed to determine the long-term health outcomes and economic implications of therapeutic interventions for type 1 and type 2 diabetes. The CDM and its validation studies have been previously described [31,32,33]. More information on CDM version 9.5 is available online (http://www.core-diabetes.com/).
Projected outcomes include incidence of complications, rates of clinical events, per patient costs, life-years (LYs) gained and quality-adjusted LYs (QALYs) gained over a lifelong time horizon (i.e. up to 50 years). Cost-effectiveness was described in terms of the incremental cost-effectiveness ratio (ICER), which is the cost per additional unit of QALY gained for the intervention versus the alternative. In the current analyses, a WTP threshold of DKK 357,100 per QALY gained was assumed (equivalent to the Gross Domestic Product per inhabitant in Denmark in 2020). Both costs and effects were discounted by 4.0% annually, according to Danish guidelines for health economic evaluation of pharmaceuticals [34]. All prices were stated in Danish krone (DKK) price-level 2020 ex VAT and relative conversions to euros (€) (Currency Converter|Foreign Exchange Rates|OANDA). All analyses were run with 1000 individuals for 1000 iterations.
Model Inputs
Clinical Data
The baseline cohort characteristics incorporated in the model were based on the data pooled from SUSTAIN 2, SUSTAIN 3, SUSTAIN 8 and PIONEER 2 reported by Capehorn et al., who evaluated the cost-effectiveness of once-weekly SC semaglutide 1 mg versus empagliflozin 25 mg for the treatment of patients with T2D [30]. The target population comprised individuals with T2D with HbA1c values between 7.0 and 10.5% (53–91 mmol/mol) uncontrolled on metformin alone. The mean age of the pooled population was 56 years, the mean duration of diabetes was 7.0 years, the mean HbA1c was 8.2%, and the mean body mass index (BMI) was 32.8 kg/m2 [30]. Baseline characteristics not reported by Capehorn et al. [35] were taken from the PIONEER 2 study. A summary of the baseline characteristics of individuals accounted in the model is provided in Supplementary Table S1. The effects of each of these drugs on physiological parameters such as HbA1c, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, HDL cholesterol and BMI were also sourced from Capehorn et al. [30]. The rates for non-severe (NSHE) and severe hypoglycaemic events (SHE1 and SHE2) were obtained from the CEA on oral semaglutide versus empagliflozin based on PIONEER 2 reported by Bain et al. [36], since no data on hypoglycaemia were reported by Capehorn et al. [30]. A summary of treatment effects is provided in Tables 1 and 2.
Treatment Intensification and Long-Term Disease Progression
All patients start a simulation by receiving either SC semaglutide plus metformin or empagliflozin plus metformin. Disease progression may be observed as a rise in HbA1c while on the same drug regimen, requiring intensification of therapy to regain glycaemic control. In the current analysis, after attaining HbA1c threshold of 7.5% (58 mmol/mol), patients switch to escalation therapy (second line [2L]). This escalation therapy is a combination of long-acting insulin glargine (0.7 IU/kg) to SC semaglutide or empagliflozin and metformin, as advised by ADA/EASD recommendations [7, 13, 14]. Reduction in HbA1c was based on an insulin-naive population derived from the 'Core' multivariate equations estimated by Willis et al. [37]. For initial treatment intensification, insulin glargine at a dose of 0.7 IU/kg was assumed for an average body weight of 91.6 kg. The HbA1c thresholds for treatment switch are in accordance with National Institute for Health and Care Excellence (NICE) guidelines [38] and clinical guidelines from the Danish Society of Endocrinology [16, 17]. Progression over time for HbA1c and blood pressure was predicted using the United Kingdom Prospective Diabetes Study (UKPDS) 68 risk equation [39]. Progression over time for other physiological parameters such as lipid levels was estimated using Framingham risk equations, available as a default option in the IQVIA CDM 9.5 model. Cardiovascular risk and mortality were calculated using the UKPDS 82 equations for CVD and the UKPDS 82 combined mortality approach equations [40]. The effect on BMI was assumed to be maintained while the patient remained on SC semaglutide or empagliflozin.
Utilities
To quantify the quality of life, CDM uses a comprehensive set of utility weightings/scores for estimating the expected QALY gain for each treatment pathway [41]. Utilities are assessed on a scale from 0 to 1, where zero represents death and one indicates a healthy person without complications. Disutilities due to illness (i.e. myocardial infarction, stroke, amputation event, ulcer, and hypoglycaemia event) are values in the range − 1 to 0 and therefore cause the quality of life utility to either decrease or remain constant. In the IQVIA CDM, quality of life is assessed for each year for each patient based on current health status, by selecting the lowest utility value when a patient experiences multiple complication (the CDM default minimum approach). Supplementary Table S2 depicts the values used for this analysis and data sources.
BMI as well as the disutility associated with BMI gain is a core component of the progression of diabetes complications over time and an important measure of the impact of treatment on patients. BMI impact on utility is estimated through inclusion of disutility based on Bagust et al. [42], assigning a disutility of –0.0061 per unit gain BMI over a BMI of 25 kg/m2.
Costs
Unit costs of treatments, including SC semaglutide and empagliflozin, were obtained from medicinpriser.dk six pricing periods (i.e. from 22 February 2021 to 3 May 2021) in accordance with guidelines for price comparisons by the Danish Medicines Agency [43]. Pharmacy purchase prices excluding VAT and pharmacy fee (in Danish: Apotekets Indkøbspriser [AIP]) were DKK 32.73 (€ 4.40) per day and DKK 11,956 (€ 1, 607) per year for 1 mg SC semaglutide and DKK 11.20 (€ 1.50) per day and DKK 4091 (€ 550) per year for 25 mg empagliflozin, respectively. Treatment costs of metformin at a dose of 1500 mg/day and of long-acting insulin, i.e. Abasaglar (DKK 0.21/U), were sourced from medicinpriser.dk. Table 3 summarises the unit costs and total annual costs of various interventions used in the model.
Costs associated with preventive interventions of diabetes complications (management costs) and direct costs for treating diabetes-related complications were obtained from Ehlers et al. [5]. More information on the input costs for clinical management and complications in the CDM model is available in Supplementary Table S3.
Analytical Approach
Base Case Analysis
The base case analysis examined the treatment with SC semaglutide or empagliflozin, in addition to metformin, until an HbA1c threshold of 7.5% was reached, following which patients underwent treatment intensification with insulin glargine (0.7 IU/kg) in addition to empagliflozin or SC semaglutide plus metformin (2L).
Scenario Analyses
As extrapolation of long-term clinical outcomes is associated with uncertainty, exploratory scenario analyses were conducted to evaluate how changes to key parameters in the modelling analyses influence the results of base case analyses. Four extra scenario analyses were therefore conducted (Supplementary Table S4).
These scenarios include evaluating the effects of 2L therapy with treatment switch occurring at HbA1c threshold of 8% and at a short time horizon of 5 years. A third scenario analysis was conducted considering a possible third line of therapy (3L) to the treatment algorithm. Patients who subsequently exceeded the HbA1c threshold of 7.5% underwent further intensification by discontinuing SC semaglutide or empagliflozin and switching to a higher dose of insulin glargine alone (0.9 IU/kg) to achieve glycaemic control. This additional analysis with 3L therapy was also assessed with treatment switch occurring at HbA1c threshold of 8%.
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis (PSA) was performed with Monte Carlo simulation method together with a non-parametric bootstrapping approach to determine parameter uncertainty around cost-effectiveness outcomes. This process involves sampling with replacement of input parameters by distribution in each bootstrap iteration of the analysis. The parameters included in the PSA are as per baseline characteristics, treatment/intervention efficacy, cost and utility data as well as transition probabilities. The sampling of parameter values represented the mean and SE as well as SD values (or 20% variation on costs) indicated in each input table (Supplementary Table S1, Table 1, and Supplementary Table S2) and determined by adequate selection of statistics distributions. Transition probabilities were sampled based on the variability of each patient’s physiological parameters and variation around the mean of risk equation’s coefficients.
Compliance with Ethics Guidelines
This cost-effectiveness study is based on the CDM model, which was used to simulate the long-term clinical and economic results of SC semaglutide and empagliflozin based on existing literature findings and completed clinical trials. Moreover, it does not involve any studies on human participants and animals directly performed by any of the authors.
Results
Base Case Analysis
The long-term projection of surrogate endpoints using the CDM model showed that, over a lifelong time horizon (50 years), individuals with T2D on SC semaglutide plus metformin gained 0.065 LYs and 0.130 QALYs at an incremental cost of DKK 96,923 (€ 13,031) compared to empagliflozin plus metformin, thereby generating an ICER of DKK 745,561 (€ 100,239) per QALY gained. In this analysis, the treatment switch occurred after the 3rd year with SC semaglutide plus metformin and after the 2nd year with empagliflozin plus metformin.
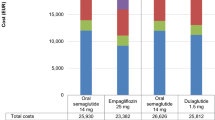
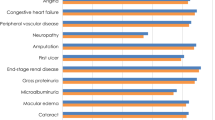
The base case analysis also showed higher treatment-related costs accruing to individuals using SC semaglutide: DKK 285,289 (€ 38,356) compared to empagliflozin DKK 181,706 (€ 24,430) (Table 4). Contrarily, costs related to disease management did not differ between the two drugs. Furthermore, no major difference in the clinical outcomes (renal disease, CVD, eye disease, ulcer, amputation, neuropathy and hypoglycaemia) was seen between the use of SC semaglutide and empagliflozin added to metformin (Supplementary Table S5).
Scenario Analyses
In a scenario where a higher HbA1c threshold of 8% was applied for treatment intensification, patients on SC semaglutide plus metformin switched to 2L therapy after the 5th year, while those on empagliflozin plus metformin switched treatment after the 4th year. In this exploration, the use of SC semaglutide plus metformin was still not cost-effective with some gains in LYs (0.073) and QALYs (0.130) at an incremental cost of DKK 98,848 (€ 13,289) compared to empagliflozin plus metformin. This was mainly attributed to the large difference in treatment cost—DKK 104,511 (€ 14,051)—between the two arms, leading to an ICER of DKK 760,369 (€ 102,230). When a time horizon of 5 years was applied, the use of SC semaglutide plus metformin was also not cost-effective, with marginal gains in LYs (0.002) and QALYs (0.041) at an incremental cost of DKK 23,916 (€ 3215) compared to empagliflozin plus metformin, leading to an ICER of DKK 583,317 (€ 78,425).
In the scenario analyses with 3L therapy, the use of SC semaglutide provided additional LYs (0.060) and QALYs (0.100) at an incremental cost of DKK 31,922 (€ 4291) generating an ICER of DKK 319,220 (€ 42,918). Assuming the WTP of DKK 357,100 (€ 48,011), this implies that SC semaglutide plus metformin reached cost-effectiveness compared to empagliflozin plus metformin. In this scenario analysis, treatment switch from 2 to 3L therapy occurred after the 4th year and the 6th year in patients with empagliflozin plus metformin and SC semaglutide plus metformin, respectively. However, in the scenario where Hb1Ac threshold was increased to 8%, treatment switch from 2 to 3L therapy occurred after the 7th year and the 8th year in patients with empagliflozin plus metformin and SC semaglutide plus metformin, respectively. In this scenario, the use of SC semaglutide provided additional LYs (0.067) and QALYs (0.105) at an incremental cost of DKK 40,971 (€ 5508) generating an ICER of DKK 390,200 (€ 52,461). Assuming a WTP threshold of DKK 357,100, this scenario indicates that SC semaglutide was not cost-effective compared to empagliflozin in T2D individuals with inadequate glycaemic control on metformin alone. This also points out the duration of therapy with SC semaglutide as the key driver of results. The results of scenario analyses are detailed Table 5.
Probabilistic Sensitivity Analysis (PSA)
Differences in costs and health outcomes between the two drugs resulting from each simulation are presented in a cost-effectiveness plane and cost-effectiveness acceptability curve (Fig. 1). The PSA analysis shows that most observations fall in the northeast (79%) and northwest (21%) quadrants of the scatterplot. The probability of SC semaglutide plus metformin as 2L therapy is cost-effective in 19% of cases compared to empagliflozin plus metformin, at the defined WTP threshold of DKK 357,100 per QALY gained [44]. However, when the hypothetical 3L therapy was considered, SC semaglutide plus metformin reached cost-effectiveness in 50% of the simulations compared to empagliflozin plus metformin at the defined WTP threshold (Fig. 1).
Cost-effectiveness scatterplots and cost-effectiveness acceptability curves: a Cost-effectiveness scatterplot for SC semaglutide vs. empagliflozin of the base case analysis using 2L treatment arm. b Cost- effectiveness acceptability curve for SC semaglutide vs. empagliflozin of the base case analysis using 2L treatment arm. c Cost-effectiveness scatterplot for SC semaglutide vs. empagliflozin of the scenario analysis using 3L treatment arm. d Cost-effectiveness acceptability curve of the scenario analysis using 3L treatment arm. DKK, Danish krone; QALY, quality-adjusted life-years; WTP, willingness to pay
Discussion
SC semaglutide does not seem to be a cost-effective treatment option versus empagliflozin for patients with T2D treated with metformin requiring treatment intensification. Treatment costs were considerably higher for SC semaglutide than for empagliflozin. The exploratory analyses pertaining to 2L therapy supported the results of the base case analyses, demonstrating that SC semaglutide plus metformin is not cost-effective compared to empagliflozin plus metformin from a Danish payer’s perspective. However, in the analyses with 3L therapy, where empagliflozin and SC semaglutide were discontinued at the time of second insulin escalation, SC semaglutide plus metformin reached cost-effectiveness compared to empagliflozin plus metformin. Note that this hypothetical analyses (3L) differs from the current international and Danish clinical guidelines, which recommend continuation of treatment with SGLT-2 inhibitor or GLP-1 receptor agonist [12,13,14, 16]. However, real-world data will confirm the extent to which these guidelines will be implemented.
The recently published CEA, which formed the basis of our current analysis [30], comparing SC semaglutide and empagliflozin, reported once-weekly SC semaglutide 1 mg to be a cost-effective treatment from a healthcare payer perspective compared with empagliflozin 25 mg for the treatment of patients with T2D with inadequate glycaemic control on metformin monotherapy in the UK. The analysis conducted by Capehorn et al. differs from ours only by the definition of next line therapy. Capehorn et al. switched from GLP-1 RA or SGLT2 inhibitor plus metformin to basal insulin alone at treatment intensification whereas in the current analyses we continued GLP-1 RA or SGLT2 inhibitor plus metformin along with basal insulin. This difference in approach reiterates the importance of the duration of therapy with SC semaglutide at the current price. In another CEA by Gorgojo-Martınez et al. [45], it was observed that SC semaglutide (0.5 mg and 1 mg) was more likely to be cost-effective versus empagliflozin (10 mg and 25 mg), irrespective of patient’s BMI at the baseline analyses in patients with inadequate glycaemic control on metformin in the Spanish setting. It should be noted that this analysis used the same approach as the analysis by Capehorn et al.
In a recent CEA conducted by Ramos et al. [7], empagliflozin plus metformin was proven to be cost-effective and even dominant (better health outcomes with lower costs) versus oral semaglutide plus metformin in the UK setting when effects on hospitalization rate due to heart failure were taken into account. That effect, compared to GLP-1 receptor agonists, was observed in a real-world study [25] and was not implemented in the current analysis.
Empagliflozin has also shown positive impact on patients with a history of CV disease [46, 47]. This resulted in a CEA performed based on the EMPA‐REG OUTCOME trial, suggesting that empagliflozin is highly cost‐effective in the UK for treatment of people with diabetes and high CVD compared to placebo [46, 47]. Also, previous work comparing empagliflozin and liraglutide has shown cost-effectiveness of empagliflozin in this population in the Danish setting [5].
There are certain limitations pertaining to the present analysis. First, the long-term cost-effectiveness results for SC semaglutide plus metformin versus empagliflozin plus metformin were based on projections of short-term outcomes on surrogate endpoints as reported in Capehorn et al. [30] based on pooled data. No direct comparisons between empagliflozin have been conducted and as such the short-term effects are based on a network meta-analysis. Also, no long-term studies have been conducted showing the impact on hard outcomes in this population with inadequate glycaemic control on metformin alone. This warrants more robust clinical trials with long-term endpoints among the comparators of interest to provide all relevant data to healthcare decision makers in maximizing healthcare benefits and disease prevention strategies. Second, hypoglycaemia values were obtained from another study with similar baseline characteristics (PIONEER 2) as reported in Bain et al. [36] since these values were not mentioned by Capehorn et al. [30]. It is, however, assumed that the rate of hypoglycaemic events is the same for oral and SC semaglutide.
Absence of long-term clinical follow-up data as one of the limitations in the study was addressed by running lifelong simulations with the CDM. It is an efficient and meaningful approach to integrate and synthesize short-term clinical trial results with data from multiple sources to inform healthcare decision-making regulatory bodies, in turn estimating the clinical outcomes and costs associated with healthcare strategies over patient lifetimes [31,32,33]. Furthermore, the scenario and sensitivity analyses conducted around the assumptions made and data inputs chosen show the robustness of the results obtained and ensure confidence in the long-term projections.
It is worth noting that the rational drug use requires that patients receive drugs appropriate to their clinical needs and that these drugs must be affordable and at the lowest cost to patients and the society [26]. Economic evaluations should be encouraged for the definition of rational drug use because they provide important information of the total costs and effectiveness of alternative treatment pathways.
Conclusion
Data from the current study suggest that SC semaglutide plus metformin showed small gains or improvements in LYs and QALYs at a high incremental cost, thereby generating an overall ICER of DKK 745,561 (€ 100,239). Based on a WTP threshold of DKK 357,100 (€ 48,011) per QALY gained, SC semaglutide plus metformin was not projected to be cost-effective versus empagliflozin plus metformin for the treatment of individuals with T2D with inadequate glycaemic control on metformin alone from a Danish payer’s perspective. At the current price of SC semaglutide, duration of therapy with SC semaglutide was found to be the key driver of results.
References
Ramos M, Men P, Wang X, Ustyugova A, Lamotte M. Cost-effectiveness of empagliflozin in patients with type 2 diabetes and established cardiovascular disease in China. Cost Eff Resour Alloc. 2021;19(1):46.
Sundhedsstyrelsen. Farmakologisk glukosesænkende behandling af type 2-diabetes i almen praksis, Rationel Farmakoterapi nr. 2020. https://www.sst.dk/da/Udgivelser/2020/Rationel-Farmakoterapi-10-2020. Accessed 10 Sep 2020.
Statens Institut for Folkesundhed. Sygdomsudviklingen i Danmark fremskrevet til 2030. 2020. https://www.sdu.dk/sif/-/media/images/sif/sidste_chance/sif/udgivelser/2017/sygdomsudviklingen_i_danmark_fremskrevet_til_2030.pdf.
Carstensen B, Rønn PF, Jørgensen ME. Components of diabetes prevalence in Denmark 1996–2016 and future trends until 2030. BMJ Open Diabetes Res Care. 2020;8:1.
Ehlers LH, Lamotte M, Monteiro S, et al. The cost-effectiveness of empagliflozin versus liraglutide treatment in people with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2021;12(5):1523–34.
Sundhedsdatastyrelsen (medstat.dk). 2018. https://medstat.dk/.
Ramos M, Cummings MH, Ustyugova A, Raza SI, de Silva SU, Lamotte M. Long-term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11(9):2041–55.
Cannon A, Handelsman Y, Heile M, Shannon M. Burden of illness in type 2 diabetes mellitus. J Manag Care Spec Pharm. 2018;24(9):S5-s13.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Rungby J, Schou M, Warrer P, Ytte L, Andersen GS. Prevalence of cardiovascular disease and evaluation of standard of care in type 2 diabetes: a nationwide study in primary care. Cardiovasc Endocrinol. 2017;6(4):145–51.
Lindvig A, Tran MP, Kidd R, Tikkanen CK, Gæde P. The economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in Denmark. Curr Med Res Opin. 2021;37(6):949–56.
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13-s27.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–8.
Cornell S. Comparison of the diabetes guidelines from the ADA/EASD and the AACE/ACE. J Am Pharm Assoc. 2017;57(2):261–5.
Association AD. Standards of medical care in diabetes—2021 abridged for primary care providers. Clin Diabetes. 2021;39(1):14–43.
Dansk Endokrinologisk Selskab. NBV: Behandling og kontrol af Type 2 Diabetes. 2020. http://www.endocrinology.dk/index.php/nbvhovedmenu/1-diabetes-mellitus/nbv-endokrinologi-behandling-og-kontrol-af-type-2-diabetes-t2d-diabetes-arskontrol-nyopdaget-diabetes-2-peroral-behandling-insulin-behandling-kolesterolbehandling-blodtryksbehandling-glp1-og-dpp4.
DSAM/DES vejledning 2018. Farmakologisk behan dling af type 2-diabetes. 2018.
Guidance for industry on diabetes mellitus-evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes; availability. 2020. https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic.
Reifsnider OS, Kansal AR, Gandhi PK, et al. Cost-effectiveness of empagliflozin versus canagliflozin, dapagliflozin, or standard of care in patients with type 2 diabetes and established cardiovascular disease. BMJ Open Diabetes Res Care. 2021;9:1.
Schnell O, Rydén L, Standl E, Ceriello A. Current perspectives on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2016;15(1):139.
Schnell O, Rydén L, Standl E, Ceriello A. Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2017;16(1):128.
Chamberlin S, Dabbs W. Semaglutide (ozempic) for type 2 diabetes mellitus. Am Fam Physician. 2019;100(2):116–7.
Optimising therapy for type 2 diabetes with empagliflozin (Jardiance). 2020. https://www.goodfellowunit.org/medcases/optimising-therapy-type-2-diabetes-empagliflozin-jardiance.
Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs. 2018;78(10):1037–48.
Patorno E, Najafzadeh M, Pawar A, et al. The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study programme: design and exposure accrual for an evaluation of empagliflozin in routine clinical care. Endocrinol Diabetes Metab. 2020;3(1):e00103.
World Health Organization (ed). Drug and therapeutics committees. 2020. https://www.who.int/medicines/technical_briefing/tbs/01-PG_DTC-Overview_final-08.pdf.
Danish Medicines Agency. Rational pharmacotherapy. 2020. https://www.sst.dk/da/opgaver/rationel-farmakoterapi.
Ehlers LH. Introduction to medical market access in Denmark. 2019. https://vbn.aau.dk/da/publications/introduction-to-medical-market-access-in-denmark.
Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 2002.
Capehorn M, Hallén N, Baker-Knight J, Glah D, Hunt B. Evaluating the cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for treatment of patients with type 2 diabetes in the UK setting. Diabetes Ther. 2021;12(2):537–55.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE del. Value Health. 2014;17(6):714–24.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5-26.
Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27-40.
The Finance Ministry. Nøgletalskatalog. 2019. https://www.fm.dk/oekonomi-og-tal/finansministeriets-regnemetoder. Accessed 19 Dec 2019.
Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–81.
Bain SC, Hansen BB, Malkin SJP, et al. Oral semaglutide versus empagliflozin, sitagliptin and liraglutide in the UK: long-term cost-effectiveness analyses based on the PIONEER clinical trial programme. Diabetes Ther. 2020;11(1):259–77.
Willis M, Asseburg C, Nilsson A, Johnsson K, Kartman B. Multivariate prediction equations for HbA(1c) lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20(3):357–71.
Type 2 diabetes in adults: management. 2020. https://www.nice.org.uk/Guidance/NG28.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no 68). Diabetologia. 2004;47(10):1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–30.
Lægemiddelstyrelsen 2018a. Vejledning om udarbejdelse af sundhedsøkonomiske analyser af lægemidler. VEJ nr 9153 af 09/03/2018 (Danish Medicines Agency. Guidelines for the development of health-economic evaluation of medicines). 2018. https://www.retsinformation.dk/Forms/R0710.aspx?id=199976.
StatBank Denmark. 2020. https://www.statbank.dk/nrs.
Gorgojo-Martínez JJ, Malkin SJP, Martín V, Hallén N, Hunt B. Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: once-weekly semaglutide versus empagliflozin. J Med Econ. 2020;23(2):193–203.
Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139(11):1384–95.
Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol. 2014;13:102.
Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13(9):814–22.
Acknowledgements
Funding
This study, including the journal’s Rapid Service and Open Access Fees, was funded by Boehringer Ingelheim.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship of this article, take responsibility for the integrity of the work and have given their approval for this version to be published.
Author Contributions
LHE, ML, MCR, SS, PH, MMK and NE conceptualized the study. LHE, ML, MCR, SS and PH performed calculations. LHE wrote the first draft. ML, MCR, SS, PH, MMK and NE provided inputs to and reviewed first draft.
Medical Writing, Editorial and Other Assistance
We would like to thank Saurabh Trikha and Paranjoy Saharia for the medical writing support.
Disclosures
Lars H. Ehlers is employed by Aalborg University, Aalborg, Denmark. His contributions to the study were supported by an honorarium funded by Boehringer Ingelheim. Mark Lamotte and Mafalda C. Ramos are employees of IQVIA, which received consulting fees from Boehringer Ingelheim for adapting the Core Diabetes Model and running the analyses. Susanne Sandgaard, Pia Holmgaard and Malene M. Kristensen are employees of Boehringer Ingelheim Denmark A/S. Niels Ejskjaer received honorarium from Boehringer Ingelheim for his contributions to this study.
Compliance with Ethics Guidelines
This article is based on the CDM model, which was used to simulate the long-term clinical and economic results of SC semaglutide and empagliflozin based on existing literature findings and clinical trials. Moreover, it does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ehlers, L.H., Lamotte, M., Ramos, M.C. et al. The Cost-Effectiveness of Subcutaneous Semaglutide Versus Empagliflozin in Type 2 Diabetes Uncontrolled on Metformin Alone in Denmark. Diabetes Ther 13, 489–503 (2022). https://doi.org/10.1007/s13300-022-01221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01221-3