Abstract
Introduction
IDegLira is a fixed-ratio combination of insulin degludec and liraglutide indicated for the treatment of type 2 diabetes (T2D). We report the first real-world study describing change in glycated hemoglobin (HbA1c) among US patients who initiated IDegLira. The aim of the study was to observe and describe changes in glycemic control and weight in patients initiating IDegLira in real-world clinical practice.
Methods
Patients in the Practice Fusion electronic medical record database who initiated treatment with IDegLira between March 2017 and June 2018 were identified (n = 1384). To be included in the analyses, the study population needed to meet age, time in database pre- and post-initiation, and availability of HbA1c data at baseline and follow-up requirements. Data were analyzed according to baseline therapy subgroups and whether patients were intensifying (primary analysis group) or simplifying (secondary analysis group) their diabetes treatment. Changes in clinical outcomes from baseline were evaluated by paired t tests and linear regression.
Results
The overall study population comprised 296 patients, of whom 206 were included in the primary analysis group and 90 were included in the secondary analysis group. In the adjusted analyses, there was a reduction in HbA1c of – 1.1% in the primary analysis group, with the HbA1c reduction in all prior therapy groups ranging from – 0.8% for those previously on basal insulin to – 1.0% for those previously on non-injectable therapy (p < 0.0001 for all). In a similar adjusted analysis, there was a statistically significant but small (1.0 lb/0.45 kg) change in weight in the primary analysis group. In the secondary analysis, patients previously on more than one injection daily switched to a more simplified therapy without compromising on glycemic control (HbA1c change of − 0.16%).
Conclusion
Consistent with previous real-world studies, IDegLira lowered HbA1c across different background prior glucose-lowering therapies, with minimal impact on weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
IDegLira is a fixed-ratio combination of insulin degludec and liraglutide indicated for the treatment of type 2 diabetes. Fixed-ratio combinations of a glucagon-like peptide-1 receptor agonist and basal insulin provide glycemic control by targeting both fasting and postprandial glucose levels with a single therapy, simplifying the treatment regimen to improve adherence. The efficacy and safety of IDegLira have been shown in several clinical trials, but real-world studies are needed to evaluate the effectiveness of IDegLira therapy in clinical practice. |
The aim of this study was to evaluate reduction in glycated hemoglobin (HbA1c) in patients who initiated IDegLira therapy in a real-world setting. |
What was learned from the study? |
Among patients intensifying therapy, IDegLira use was associated with a statistically significant mean reduction of – 1.1% in HbA1c at 6 months compared with baseline. |
Among patients simplifying therapy, HbA1c did not deteriorate despite a reduction in daily injections of antidiabetic medication. |
Introduction
According to the US Centers for Disease Control and Prevention, 34.2 million people in the USA had diabetes in 2018 (~10.5% of the US population), and the total of the estimated direct and indirect costs associated with diabetes in 2017 was US$327 billion, highlighting the significant economic burden of this disease [1, 2]. Use of a combination of basal insulin (BI) and glucagon-like peptide-1 receptor agonist (GLP-1 RA) therapy for glycemic control in type 2 diabetes (T2D) is well established [3,4,5,6,7] and recommended by the latest American Diabetes Association (ADA) and American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) clinical practice guidelines for the management of T2D [8, 9].
IDegLira is a fixed-ratio combination of insulin degludec (IDeg) and the GLP-1 RA liraglutide (Lira) that is indicated for the treatment of T2D. The efficacy and safety of IDegLira has been evaluated in an extensive phase 3 clinical trial program (DUAL trials), which enrolled patients with T2D and inadequate glycemic control on either oral antidiabetic drugs (OADs), GLP-1 RA therapy, BI therapy, or sodium-glucose cotransporter-2 inhibitors [10,11,12,13,14]. By design, randomized controlled trials (RCTs) are restricted to patients meeting strict inclusion and exclusion criteria who are likely not to be representative of usual care settings, so it is important to evaluate the benefit of IDegLira in patient populations more representative of usual care. In general, patients seen in day-to-day clinical practice tend to be older, have higher body weight and levels of comorbidity, and be less adherent to treatment than those enrolled in RCTs; thus, they could potentially derive multiple benefits from a simple combination therapy in terms of glycemic control, weight management, and cardiovascular risk [15].
However, real-world evidence on the effectiveness of IDegLira is limited. To date, real-world results for a 6-month follow-up have been published from three studies: two in Europe and one in Israel. No retrospective studies conducted in the USA have yet been published, although characteristics of patients on IDegLira in the USA have been reported recently [15]. The first real-world European study, which included a relatively small sample size of 61 patients from a single Swiss medical center [16], showed a mean reduction in glycated hemoglobin (HbA1c) of –1.7%, which is well within the range of reduction observed in the 26-week DUAL trials (–1.4 to –1.9%) [10,11,12,13,14]. The second European study, which included 611 patients, and the study in Israel, which included 413 patients, reported reductions of –0.9 and −0.65% in HbA1c, respectively [17, 18].
Large clinical databases that extract data collected through electronic medical record (EMR) systems allow for the review of treatment patterns and clinical effectiveness among patients treated in usual care settings across multiple sites. The aim of this study was to analyze data from EMRs to evaluate clinical parameters in patients with T2D initiating IDegLira treatment. This is the first real-world study describing change in HbA1c among T2D patients initiating IDegLira in the USA; it also contributes to the body of real-world evidence by describing outcomes in both specialty and primary care settings.
The objectives of this study were to observe and describe changes in glycemic control and weight in patients who had initiated IDegLira in real-world clinical practice. Changes in glycemic control and weight were analyzed according to prior antidiabetic therapy.
Methods
Study Design and Data Source
This retrospective, observational study used de-identified data from the Practice Fusion EMR database. The Practice Fusion ambulatory EMR platform is currently in use at over 25,000 clinical sites, mostly single-provider or small group practices, in all 50 US states, representing approximately 6% of all ambulatory care among primary care and specialist practices in the USA [19]. The Practice Fusion EMR patient population is comparable to the overall US population in terms of age, gender, and geographic location [20]. Key data elements captured by the system include demographics, prescription data (National Drug Codes), diagnosis data (International Classification of Diseases, Ninth [Tenth] Revision, Clinical Modification [ICD 9-CM/10-CM] codes), laboratory test results (Logical Observation Identifiers Names and Codes), physician characteristics, office visits, and vitals [21]. The resulting EMR data are made available for research in the form of a certified Health Insurance Portability and Accountability Act-compliant de-identified research database.
As a non-interventional, retrospective study analyzing a de-identified dataset, this study did not require approval by an institutional review board or patient informed consent.
Patient Selection
Eligible patients had a prescription order for IDegLira entered in the electronic health record (EHR) platform from the time of US launch (March 2017) to June 2018, with a previous diagnosis of T2D (ICD-9-CM diagnostic code 250.x0 or 250.x2, or ICD-10-CM diagnostic code E11) or a prescription record for insulin. The index date was set as the date of the first qualifying IDegLira prescription order. As only year of birth was available, to ensure all patients were adults, eligible patients were aged ≥ 19 years at the time of initiation on therapy with IDegLira and did not have a prior diagnostic code for type 1 diabetes. Patients were required to have ≥ 90 days of time prior to index date and ≥ 135 days of time post-index in the EMR database, as defined by the presence of an office visit, prescription order, or laboratory test. Patients who were pregnant or had gestational diabetes 9 months prior to the index date and up to 135 days after the index date were excluded. The final study cohort was then limited to patients with both an HbA1c value in the 6-month period before the first prescription of IDegLira and in a window of ± 45 days around the 6-month post-index time point (Fig. 1).
Patients meeting these criteria were assigned to one of six baseline regimen subgroups, based on medications reported in the 180-day baseline period prior to the index date. The primary analysis group consisted of patients in the final study cohort who were intensifying their baseline treatment regimen from any one of three baseline treatments (basal insulin alone; GLP-1 RA alone; no injectable therapy ± OADs). The secondary analysis group consisted of three additional subgroups of patients in the final study cohort who were simplifying their prior therapy regimen from more complex combinations of medications (e.g., multiple daily injections [MDI] ± OADs; GLP-1 RA with basal, bolus, or premix insulin; and bolus or premix insulin only). Finally, patients without a stop date for IDegLira entered into the EMR system within 135 days of initiation were assumed to have continued treatment and were designated as IDegLira-continuing patients.
Baseline Clinical Characteristics and Outcome Measures
Baseline clinical characteristics included prescribing physician specialty, therapy, and comorbidity with calculated scores for the Charlson Comorbidity Index (CCI) and the Diabetes Complications Severity Index (DCSI) [22, 23]. Outcomes of interest included change in HbA1c and weight. Outcomes were assessed at 6 months post-index, using the value that was closest to 180 ± 45 days following the index date. Change in both HbA1c and weight was defined as the absolute difference from baseline to follow-up, with baseline measured as the value that was closest to the index date, including up to 7 days post-index date but no more than 180 days prior to index date.
Statistical Analyses
Descriptive statistics were calculated, with patient number and percentage reported for categorical variables, and mean, standard deviation (SD), median, and range reported for continuous variables. Statistical analyses were performed for change in HbA1c and weight for the primary and secondary analysis groups, for all patients, and for the six prior therapy groups individually. Mean difference between baseline and follow-up was tested using a paired t test. Statistical significance was set at p < 0.05. Linear regression models were run for mean individual change in HbA1c and weight from baseline to follow-up. Statistically significant demographic or baseline covariates were entered into the models by stepwise forward selection. In addition, clinical outcomes in a subset of IDegLira-continuing patients were evaluated and compared to those of the overall study population. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Demographic and Clinical Characteristics of the Study Population
Of the 1384 patients newly prescribed IDegLira during the study period, 596 fulfilled other inclusion criteria and had a baseline HbA1c (–180 to +7 days) available (Fig. 1). Of these, 296 patients also had a follow-up HbA1c measure (180 ± 45 days) available. The final study cohort comprised the 296 patients for whom both baseline and follow-up HbA1c values were available. A comparison of the 296-patient study cohort to 300 patients excluded from the final cohort due to HbA1c values not being available at follow-up (baseline HbA1c-only group) showed that the two groups were similar with respect to most baseline clinical characteristics evaluated, including weight, body mass index (BMI), HbA1c, and comorbidities (p > 0.05 for all); the exceptions were gender (57.1 vs 47.7% male; p = 0.02) and provider specialty (59.8 vs 70.7% primary care; p = 0.01) for study population versus baseline HbA1c-only groups, respectively. The breakdown by prior therapy subgroup was as follows: BI alone (n = 56; 18.9%); GLP-1 RA alone (n = 49; 16.6%); no injectable therapy (n = 101; 34.1%); MDI (n = 29; 9.8%); GLP-1 RA with basal, bolus, or premix insulin (n = 54; 18.2%); and bolus or premix insulin only (n = 7; 2.4%). There were 241 patients who had weight recordings at both baseline and follow-up.
Baseline demographic and clinical characteristics are presented by prior therapy subgroup in Table 1. The mean (SD) age of study patients was 60.2 (10.8) years, and the majority were male (57.1%). A majority of index IDegLira prescriptions were written by primary care providers (59.8%), ranging from 43% in the bolus or premix-only subgroup to 67% in the GLP-1 RA-only subgroup. The mean CCI and DCSI scores in the overall study population were 2.2 (1.8) and 1.5 (2.4), respectively; the GLP-1 RA group had the lowest CCI and DCSI scores: 1.9 (1.4) and 1.0 (1.8), respectively. The overall proportion of patients with a comorbid diagnosis of hypertension was 72.3%, and the proportion of patients with a comorbid diagnosis of dyslipidemia was 74.0% (Table 1).
The mean (SD) baseline HbA1c for all patients was 8.7% (1.9%), ranging from 7.3% (1.4%) in the bolus or premix-only subgroup to 9.2% (1.5%) in the no injectable therapy subgroup. The mean follow-up HbA1c for all patients was 8.1% (1.7%), ranging from 7.1% (1.3%) in the bolus or premix-only subgroup to 8.3% (1.8%) in the MDI subgroup. The mean baseline weight for all patients was 216.6 (53.5) lb (98.5 kg), and the mean baseline BMI was 34.5 (7.2) (Table 1).
Clinical Outcomes
In the overall study population, consisting of all six subgroups, there was a reduction in HbA1c of −0.68% from baseline to follow-up. In the primary analysis group, which consisted of the three subgroups of patients intensifying their baseline treatment regimen, there was an overall reduction in HbA1c of −0.91% from baseline to follow-up. A reduction was also observed in each of the three subgroups individually (−0.71, −0.91, and −1.02%, for the BI alone, GLP-1 RA alone, and no injectable therapy subgroups, respectively). In the secondary analysis group, which consisted of three subgroups of patients simplifying their baseline treatment regimen, reduction in HbA1c was less than that in the primary analysis subgroup, both overall (−0.16%) and in all three baseline regimen therapy subgroups: MDI (−0.03%); GLP-1 RA with basal, bolus, or premix insulin (−0.22%); and bolus or premix insulin only (−0.24%).
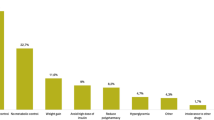
In a linear regression analysis, the adjusted mean change in HbA1c from baseline to follow-up for the primary analysis group showed a statistically significant reduction of −1.1% after adjustment for age, gender, provider specialty, HbA1c at baseline, BMI at baseline, weight at baseline, cholesterol at baseline, hypertension, dyslipidemia, CCI, DCSI, number and type of OADs at baseline, and use of basal insulin or GLP-1 RA at baseline (Fig. 2). Similarly, there was an HbA1c reduction in all three subgroups individually: −0.81% for those previously on basal insulin, −1.01% for those previously on GLP-1 RA, and −1.03% for those previously on non-injectable therapy (p < 0.0001 for all).
Mean change in HbA1c by prior therapy subgroup. Mean change was adjusted for age, gender, provider specialty, HbA1c at baseline, body mass index (BMI) at baseline, weight at baseline, cholesterol at baseline, hypertension, dyslipidemia, Deyo–Charlson Comorbidity Index (CCI), Diabetes Complications Severity Index (DCSI), number and type of OADs at baseline, and use of basal insulin or a GLP-1 RA at baseline. There were three prior therapy subgroups in the primary analysis group (patients intensifying their treatment from basal insulin alone; from GLP-1 RA alone; or those who had no injectable therapy) and three in the secondary analysis group (patients simplifying their treatment from MDI; from GLP-1 RA with basal, bolus, or premix insulin; or from bolus or premix insulin alone). The no injectable therapy and MDI subgroups were with or without OAD. bindicates that change in HbA1c from baseline was statistically significant within the subgroup (p < 0.0001). Error bars represent 95% confidence intervals (CIs). GLP-1 RA Glucagon-like peptide-1 receptor agonist, MDI multiple daily injections, n number of patients, OAD oral antidiabetic drug
The mean follow-up weight (SD) for all patients was 216.8 (52.8) lb (98.6 kg) and the mean BMI for all patients at follow-up was 34.5 (7.1) kg/m2 (Table 2). In a linear regression analysis, there was a small, statistically significant weight gain (1.04 lb/0.47 kg; p < 0.005) in the primary analysis group. This was due to weight gain in both the GLP-1 RA (+2.96 lb/1.35 kg) and the no injectable therapy (+1.51 lb/0.69 kg) prior therapy subgroups, despite weight loss in the BI alone subgroup (−1.47 lb/0.67 kg) (p < 0.005, p < 0.05, and p < 0.005, respectively) (Fig. 3).
Mean change in weight by prior therapy subgroup. Mean change was adjusted for age, gender, provider specialty, HbA1c at baseline, BMI at baseline, weight at baseline, cholesterol at baseline, hypertension, dyslipidemia, CCI, DCSI, number and type of OADs at baseline, and use of basal insulin or a GLP-1 RA at baseline. See caption to Fig. 2 for explanation of prior regimen subgroups. b and c indicates that change in weight from baseline was statistically significant within the respective subgroup (p < 0.005). Error bars represent 95% CIs
Patients Continuing IDegLira
In our initial analysis, the use of IDegLira was assumed to begin for all 296 patients on their first recorded start date and to continue for the duration of the 180-day follow-up period. In a further analysis, 124 patients who had a stop date for IDegLira entered in the EHR within 135 days of initiation were considered to have discontinued IDegLira and were removed from the analysis, leaving 172 patients for whom there was greater confidence that use of IDegLira continued throughout the entire follow-up period. In the primary analysis group, reduction in HbA1c was greater in the subset of patients continuing IDegLira compared to those with a stop date within 135 days (−1.1 vs −0.9%, respectively), with increased reductions observed in all three subgroups of patients continuing IDegLira (BI alone, GLP-1 RA alone, and no injectable therapy: −1.1, −1.0, and −1.2%, respectively). These results suggest that our initial analysis included a number of patients who may not have taken IDegLira long enough to realize its full benefit.
Discussion
This study is the first observational study on real-world initiation and effectiveness of IDegLira for the treatment of T2D in the USA. Our findings are consistent with those of both RCTs of IDegLira use and the few real-world observational studies that have previously been done. The real-world patients in our study are mostly seen at small independent practices in community settings. The patients seen at these practices tend to be older, with a higher body weight and higher baseline HbA1c levels than patients participating in RCTs [10,11,12,13,14]. Comorbidity burden also tends to be greater in real-world populations [24].
Our overall result of a −0.84% reduction in HbA1c, with −1.1% observed in the primary analysis subgroup, is similar to the −0.9% observed overall in a similar real-world study conducted in multiple European countries by Price et al. [17] and shows a greater reduction than the −0.65% observed in the Melzer-Cohen et al. study conducted in Israel [18]. Furthermore, despite different definitions of the baseline therapy groups, we see a similar pattern of HbA1c reduction in our study and in that of Price et al. [17]. The non-injectable therapy group in both studies had the largest observed reduction in HbA1c. A similar pattern of weight change was also observed in the current study and in the study by Price et al. [17]. While overall weight change was not statistically significant, we observed a small statistically significant increase in weight (+1.04 lb/0.47 kg) in the primary analysis group, driven by a statistically significant increase in weight (+2.96 lb/1.35 kg) in the GLP-1 RA prior therapy subgroup. A weight change of −4.81 lb (2.19 kg) in the MDI prior therapy subgroup was also observed. Comparable weight change has been observed in similar baseline therapy groups in the Price et al. study, reinforcing a pattern of little to no weight change for patients initiating IDegLira [17].
A strength of the current study is the size and coverage of the EMR data source provider network. However, the source network is primarily small, independent practices, and results may not be generalizable to other care settings. The results are also timely given that the data source only has a 1-month lag from time of entry until data are made available for research studies.
Our study findings should also be considered within the context of study design and limitations inherent in EMR data collection. As this was a retrospective, observational study, potential confounding effects due to unmeasured variables, such as diet, lifestyle, and medication adherence, which were not assessed in this study, cannot be ruled out. Data captured in an EMR system reflect routine clinical practice rather than mandatory assessments at pre-specified time points, which may have an impact on the quality and timing of available data. Available data may still be incomplete due to lack of documentation of patient care. In addition, our drug exposure definition is based on written prescription orders, with no confirmation of whether the prescription was filled at the pharmacy or taken by the patient. We have confidence that EHR prescription orders reflect actual use. In an analysis of data from patients continuing IDegLira, as evidenced by no medication stop date entered in their record, HbA1c reduction was greater than in patients for whom such a date was entered. However, our exposure definition is still limited by the fact that we were not able to evaluate IDegLira dose, as units of insulin are not entered into the EMR system in a consistent manner.
Conclusions
Consistent with findings reported in previous real-world studies, our results show that IDegLira can be used as an intensification strategy for patients across a range of different baseline therapies. HbA1c was lower in patients after initiation of IDegLira, with minimal impact on weight, while offering a simplified therapy for patients previously on more than one injection daily. Among patients simplifying therapy, HbA1c did not deteriorate even though many patients reduced the number of medications they were taking.
References
Centers for Disease Control and Prevention. National diabetes statistics report, 2020. 2020. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 6 Mar 2020.
American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41:917–28.
Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36:2489–96.
Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013;36:2497–503.
Ahmann A. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17:1056–64.
Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16:636–44.
Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37:2763–73.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:90–102.
Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107–39.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93.
Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101–14.
Lingvay I, Perez Manghi F, Garcia-Hernandez P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898–907.
Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41:1009–166.
Philis-Tsimikas A, Billings LK, Busch R, et al. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add-on to sodium-glucose co-transporter-2 inhibitor therapy: a randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:1399–408.
Mocarski M, da Rocha Fernandes J, Radin M, et al. Current type 2 diabetes mellitus injectable intensification patterns and IDegLira in US real-world practice. Poster presented at the AMCP Managed Care and Specialty Pharmacy Annual Meeting. Boston; April 23–26, 2018 *(poster E3)
Sofra D. Glycemic control in a real-life setting in patients with type 2 diabetes treated with IDegLira at a single Swiss center. Diabetes Ther. 2017;8:377–84.
Price H, Bluher M, Prager R, et al. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20:954–62.
Melzer-Cohen C, Chodick G, Naftelberg S, Shehadeh N, Karasik A. Metabolic control and adherence to therapy in type 2 diabetes mellitus patients using IDegLira in a real-world setting. Diabetes Ther. 2020;11:185–96.
IQVIA. Physician office usage of electronic health records software: market insights report. 2018. https://www.iqvia.com/-/media/iqvia/pdfs/us-location-site/commercial-operations/iqvia-ehr-adoption_2018.pdf?_=1567197241783. Accessed 6 Mar 2020.
Practice Fusion. Comparison of the Practice Fusion EHR Database to the National Ambulatory Medical Care Survey (NAMCS). San Francisco: Practice Fusion; 2016.
Practice Fusion. Practice Fusion EHR Database, data dictionary. San Francisco: Practice Fusion; 2017.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23.
Mocarski M, Da Rocha Fernandes J, Radin M, et al. Current type 2 diabetes mellitus injectable intensification patterns and IDegLira in US real-world practice. J Manag Care Spec Pharm. 2018;24(Suppl):S38.
Acknowledgements
Funding
This study and the Rapid Service Fee were funded by Novo Nordisk A/S.
Editorial Assistance
The authors are grateful to Helen Marshall of Watermeadow Medical, an Ashfield company, for editorial support, funded by Novo Nordisk.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Prior Presentation
Partial results were presented in poster sessions at both the American Diabetes Association (ADA) 2019 and Canadian Diabetes Association (CDA) 2019 meetings.
Disclosures
Alina Bogdanov, Lauren Fischer and Lee Kallenbach are employees of Veradigm Health, under contract with Novo Nordisk A/S for the conduct of the study. João Diogo Da Rocha Fernandes is an employee of Novo Nordisk A/S. Leonard E. Egede has nothing to disclose.
Compliance with Ethics Guidelines
As a non-interventional, retrospective study analyzing a de-identified dataset, approval by an institutional review board and patient informed consent were not necessary.
Data Availability
The data that support the findings of this study are available from Veradigm Health. Restrictions apply to the availability of these data, which were used under license for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features To view digital features for this article go to: https://doi.org/10.6084/m9.figshare.12378110
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Egede, L.E., Bogdanov, A., Fischer, L. et al. Glycemic Control Among Patients Newly Prescribed IDegLira Across Prior Therapy Group in US Real-World Practice. Diabetes Ther 11, 1579–1589 (2020). https://doi.org/10.1007/s13300-020-00850-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00850-w