Abstract
Introduction
End-stage renal disease (ESRD) is associated with increased cardiovascular mortality (CVM) and diabetes mellitus (DM), which in many cases is treated with insulin. Skin lipohypertrophy (LH) very often occurs in insulin-treated (IT) patients as a consequence of inadequate injection technique and is one of the most prominent contributors to hypoglycemia (HYPO), glycemic variability (GV), and poor metabolic control (PMC).
Method
The aim of our multicenter observational study was to assess LH prevalence at self-injection sites and any possible factors predicting high LH/HYPO rates and GV in 296 dialyzed ITDM patients characterized by 64 ± 7 years of age, 7 ± 2 years disease duration, 2.6 ± 2.2 years dialysis duration, preferred pen utilization (80%), and basal-bolus regimen (87.4%) with self-injections (62.6%) largely surpassing caregiver-assisted ones (16.9%), and a mix of the two injection methods (20.5%).
Results
LH was detected in 57% of patients. Univariate analysis followed by backwards stepwise multivariate logistic regression function showed increased odds for developing LH in patients characterized by needle reuse, smaller injection areas, missed injection site rotation, higher HbA1c levels, and more prominent rates of HYPO and GV.
Conclusion
This was the first time such observation was made. It is now time for further studies aimed at providing evidence also in ESRD ITDM patients for the cause–effect relationship among wrong injection behavior, LH, and poor metabolic control and for the long-term preventative role of suitable educational countermeasures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin has always been considered as the treatment of choice in dialyzed DM patients but is often responsible for hypoglycemic events requiring a substantial dose reduction over time [1, 2]. In fact, despite being mostly asymptomatic, hypoglycemia (HYPO) occurs quite often in dialyzed people as a result of various factors including not only oral hypoglycemic agents or insulin but also dietary errors/prolonged fasting/alcohol intake/chronic malnutrition, malignancies, heart/hepatic/real failure, adrenal or thyroid deficiency, beta-blockers, or other drugs [3]. Moreover, nephrologists know very well that large intra-/between-day glycemic variability (GV) results from intermittently enhanced insulin clearance rate in end-stage renal disease (ESRD) patients further increasing HYPO-related cardiovascular risk (CVR) [4,5,6,7,8,9].
Another factor eventually contributing to HYPO and GV rate and adding to comorbidities typically found in dialyzed patients might be the presence of insulin-related skin lipohypertrophy (LH) [10,11,12,13,14,15]. Indeed LH has been shown to deteriorate both quality of life [11] and glycemic control [16, 17] despite increased insulin dosage [11], thus raising costs [30] as a result of higher acute/chronic complications and emergency room access rates [12, 18, 19].
The aim of the study was to assess LH rate as primary outcome and, as secondary outcome, to identify any possible factors predicting high LH/HYPO rates and GV in dialyzed insulin-treated patients.
Methods
This multicenter observational study focused on LH identification at insulin self-injection sites in a large series of dialyzed DM patients. The dialysis units (DUs) contributing to the study as the Nefrocenter Research Network (Campania Region, Southern Italy) followed standardized procedures within the frame of a shared care pathway and used the same software for data recording.
Enrolment Criteria
All DM outpatients over 17 years old on multiple daily insulin (MDI) regimen for at least 1 year referred to our DUs were consecutively enrolled.
Exclusion Criteria
Exclusion criteria were neoplastic diseases, advanced liver disease, and steroid treatment.
Study Protocol
The protocol was designed according to the Helsinki declaration and approved by the Ethics Committee of Vanvitelli University, Naples, Italy (Trial registration number 126-11.01.2019). All patients provided informed consent to personal data utilization for research purposes and completed a validated questionnaire-based interview with the help of specifically trained nurses [20,21,22,23] by also indicating all sites used for insulin injection. After that, medical investigators checked those sites for the presence of skin lesions according to a structured protocol and, in order to ensure subsequent analysis under strictly anonymous conditions, filled in a web-based clinical record form (eCRF) identifying patients by a unique ID key and uploading to a database matching clinical data with questionnaire answers.
Recorded parameters were demographics; diabetes duration; daily insulin shot number and dose; ice-cold insulin utilization; pen/syringe injection use; needle features (length/gauge) and reuse rate; size, rotation rate and relative distance of injection sites; glycemic variability and eventually occurring injection into LH lesions; unexplained hypoglycemic events as previously described [20].
Specific Details
-
1.
HYPO was defined according to American Diabetes Association (ADA) statements [3], i.e., the occurrence of one or more symptoms of hypoglycemia (including palpitations, tiredness, sweating, hunger, dizziness, and tremor) and a confirmed blood glucose (BG) reading ≤ 70 mg/dL. Frequent unexplained hypoglycemia was defined as having HYPO at least once a week in the absence of any identified precipitating event including changes in insulin dosage, diet composition, or amount of physical activity. HYPOs were further defined as severe (SH; BG < 50 mg/dL) and non-severe (NSH; 50 > BG < 70 mg/dL) [23,24,25,26].
-
2.
GV was investigated by a validated questionnaire already used in previous studies [11, 27, 28] and, as a result of the lack of universally accepted criteria, was defined as unpredictable and unexplained shifts from < 60 mg/dL to > 250 mg/dL occurring one or more times on the same day or on different days within 1 week during the previous month [11, 27, 28]. Since a large glycemic variability is typically observed between days on and off dialysis, the average of the widest glycemic variations occurring on three dialysis days and three dialysis-free days within 1 week was considered.
The evaluation–validation of HYPOs and GV was based on the analysis of patients’ self-monitored blood glucose (SMBG) recordings using a twice-a-day “staggered” protocol as previously described [12], and, according to a specific pre-study training, of any additional glucose checks performed in case of sudden HYPO symptoms. This highly flexible method was chosen as it is strongly supported by specialists owing to its easier and immediate interpretation and it is easily accepted by patients, thus ensuring the strictest possible adherence to given instructions [29].
Lipohypertrophy
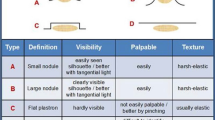
Two trained physicians for each dialysis unit separately identified LH at all sites utilized by insulin-treated patients and, in case of any discrepancy, did it again until final agreement. The LH identification procedure referred to a structured observation and palpation method [30, 31] taking into account further suggestions derived from previously published experience [32]. Briefly, the method consisted of the inspection of each area of interest through direct and tangential light against a dark background and of careful palpation implying slow circular and vertical, progressively deeper finger tip movements followed by repeated horizontal attempts on the same spot. In the present study only LH lesions were considered.
Statistical Analysis
Predictors of LH were identified by sequentially examining data with SPSS version 20. A p value less than 0.05 was considered as statistically significant. Contingency tables showed no low expected counts (< 5 for 20% of cells) for categorical variables, confirming Pearson’s chi-square test as reliable. Mann–Whitney U test and Pearson chi-square test were first performed to assess whether or not variables under investigation displayed any different mean levels/percentage distribution in patients with LH (LH+) as compared to those without LH (LH−). Then all variables were entered into unadjusted odds ratio analysis according to univariate binary logistic regression function and significant ones were then removed one at a time from subsequent stepwise backward multivariate logistic regression analysis as suited to no longer significant elements. After calibration and discrimination ability assessment by Hosmer–Lemeshow goodness of fit test (HLGOF) and receiver operating characteristic (ROC) analysis, respectively, adjusted odds ratios were reported as estimated in the refitted final model.
Results
Thirty-two specialized structures took care of 1004 adult dialyzed outpatients, of whom 371 had DM and 296 were enrolled in the study (18 with T1DM; 6.1%) (Fig. 1) for being insulin-treated (202 injected insulin four times a day and 94 three times a day, respectively); their main features are summarized in Tables 1–3: 64 ± 7 years of age, 7 ± 2 year disease duration, 2.6 ± 2.2 year dialysis duration, preferred pen utilization (80%), and basal-bolus regimen (87.4%) with self-injections (62.6%) largely surpassing caregiver-assisted ones (16.9%), and a mix of the two injection methods (20.5%).
No lipoatrophic lesions (LAs) were found. A total of 169 patients (57.1%) had LH lesions, of which 12 occurred at only one injection site, 52 at two, and all the others at multiple sites, most frequently represented by the abdomen (35%), followed by arms (30%), thighs (25%), and buttocks (10%). In total 88.5% admitted using LH areas for insulin shots and surprisingly 21.3% of patients had lesions at sites not recommended for injection, including immediately above the knee (n = 24), just below the free groin (n = 8) as well as at proximal (n = 12) and distal forearm (n = 2). A total of 127 patients had no LHs (42.9%).
On the basis of univariate analysis, LH+ patients were similar to LH− patients in terms of age, diabetes or dialysis duration, needle length, insulin total daily dose (TDD), number of injections/day, and BMI (Tables 2 and 3), while statistically significant associations were found by multivariate analysis (p < 0.05 to < 0.001) among LH and smaller injection area, missed injection site rotation, needle reuse, higher HbA1c values, larger glycemic variability, and more frequent HYPOs (severe/non severe) (Tables 3 and 4).
Discussion
Subcutaneous insulin absorption is one of the key factors affecting glycemic control in DM patients on insulin therapy. ESRD is most often associated with DM requiring insulin. Subcutaneous LH has been reported to impair regular insulin absorption and thus lead to poor glycemic control with frequent unexplained HYPOs and large GV, both of which result into acute and chronic complications and poor quality of life representing a major disease burden [11]. Poor injection habits may also affect ESRD patients’ LH and thus increase their inherent severe cardiovascular risk through impaired metabolic control.
To our knowledge this is the first study addressing this problem. It points out that over 50% of ESRD patients have LH due to injection technique errors including missed injection site rotation, selection of small skin areas and long needles, needle reuse, and ice-cold insulin. Should we try to compare our results with those made on non-ESRD DM patients, we would face problems due to the huge variability (1.9–73.4%) of reported LH rate, which in a previous paper we suggested depended on large differences in identification methods [32]. Indeed some authors relied on self-administered patient questionnaires, others on ultrasonography, and some on investigators with poorly specified training in the field if any [32]. For instance, Patil et al. found that “twenty-five (11.1%) subjects had noticed persistent swelling at their injection sites suggestive of the LH”, which in fact might not reflect actual LH presence [33]. Conversely, in a series of 387 people with T2DM [10], LH prevalence was as high as 77% according to a structured LH investigation method utilized by well-trained health professionals already successfully involved in LH-related studies [30, 31]. In addition to that, as pointed out by some authors, LH prevalence among studies may vary depending on lack of routine skin examination in diabetes clinics and/or on missed adherence to validated methods by poorly experienced health professionals [34, 35]. We recently published three papers dealing with best practice in LH identification which, indeed, is strongly hampered by the huge differences in lesion size, texture, and protrusion above the skin [15, 30,31,32] and underlined the importance of both operator experience and LH features. Despite usually being large and protruding, lesions may also take the appearance of flat, hardly visible subcutaneous tissue thickening spots which only careful and trained professionals can detect. Therefore, the poor worldwide LH identification rates call for appropriate education and refresher courses to be regularly organized in order to get wrong injection techniques corrected and thus reduce the often underestimated yet invariably deleterious metabolic and economic LH consequences [14, 18, 36].
Limitations
We are well aware that being the first study addressing this issue implies the absence of any previously published data on LH rate to compare with, which indeed can be considered a major limitation per se. Another limitation is its cross-sectional observational design, which, as opposed to what has already been shown in DM patients without ESRD, allows us only to outline correlations linking LH rate to poor metabolic control indices without defining any clear cause–effect relationship. Because of that we have already launched a follow-up study to provide confirmation for that in this specific population.
Conclusions
In any case, LH rate and metabolic consequences observed in this study agreed with those we had already described in non-dialyzed insulin-treated DM patients, with special reference to the close association of LH lesions with poor metabolic control as expressed by elevated HbA1c values and by high HYPO and GV rates [12, 14, 16, 18, 30, 32]. Of particular interest is the extent of glycemic variability as an average of the days on and off dialysis. Education on correct injection techniques has been reported to improve GV in diabetic patients with LH [11, 37, 38]. Should education prove to be able to reduce GV depending on poor injection technique also in patients on dialysis, glycemic differences observed on days on and off dialysis could be reduced too, thus providing patients with significant clinical advantages and the medical staff with better overall performance.
All this makes us consider LH as a further complication in these patients, already burdened by threatening cardiovascular consequences.
Moreover, since unexplained and unpredictable HYPOs and large GV are independent factors of increased acute cardiovascular risk, hospitalization, and all-cause mortality [5], all health professionals working in dialysis units should be trained in preventing LH development and progression and eventually aid in LH regression to try and eliminate the additional metabolic risk burden to the already high ESRD-related quality of life disrupting potential.
Our paper paves the way for further cost analysis investigations taking into account insulin administration technical errors first and any possible positive effects of highly focused patient education courses, which are in fact ongoing at the moment by the Nefrocenter Research Study Group.
References
Arnouts P, Bolignano D, Nistor I. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transpl. 2014;29(7):1284–300.
Guideline development group. Clinical practice guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR < 45 mL/min). Nephrol Dial Transplant. 2015;30:1–142.
Adrogué HJ. Glucose homeostasis and the kidney. Kidney Int. 1992;42(5):1266–82.
Xia J, Yin C. Glucose variability and coronary artery disease. Heart Lung Circ. 2019;28(4):553–9. https://doi.org/10.1016/j.hlc.2018.10.019.
Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221–30. https://doi.org/10.1016/S2213-8587(18)30136-0.
Zhou JJ, Schwenke DC, Bahn G, Reaven P. VADT Investigators Glycemic Variation and Cardiovascular Risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2018;41(10):2187–94. https://doi.org/10.2337/dc18-0548.
Lo SC, Kornelius E, Huang JY, et al. Early cardiovascular risk and all-cause mortality following an incident of severe hypoglycaemia: a population-based cohort study. Diabetes Obes Metab. 2019;11:5–9. https://doi.org/10.1111/dom.13746.
International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019. https://doi.org/10.1016/s2213-8587(18)30315-2.
Park HK. Severe hypoglycemia and cardiovascular disease in type 2 diabetes. Diabetes Metab J. 2015;39:478–80. https://doi.org/10.4093/dmj.2015.39.6.478.
Strollo F, Guarino G, Armentano V, et al. Unexplained hypoglycaemia and large glycaemic variability: skin lipohypertrophy as a predictive sign. Diabetes Res Open. 2016;2(1):24–32. https://doi.org/10.17140/droj-2-126.
Blanco M, Hernandez MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–53.
Gentile S, Strollo F, De Rosa N, et al. Injection-related local side effects in the treatment of diabetes mellitus: a methodological approach and possible solutions. Consensus statement of AMD-OSDI study group on injection technique. Dover, DE: SM Group.
Gentile S, Ceriello A, Strollo F. Insulin shot dependent lipodystrophy: evidence, uncertainties and current terminology overlaps. J Diabetes Metab Disord Control. 2016;3(3):00067. https://doi.org/10.15406/jdmdc.2016.03.00067.
Gentile S, Strollo F, Ceriello A. Lipodistrophy and associated risk factors in insulin-treated people with diabetes. Int J Endocrinol Metab. 2016. https://doi.org/10.5812/ijem.33997.
Gentile S, Strollo F, Ceriello A, AMD-OSDI Injection Technique Study Group. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: are we sure everything is clear? Diabetes Ther. 2016;7(3):401–9. https://doi.org/10.1007/s13300-016-0187-6.
Gentile S, Agrusta M, Guarino G, et al. Metabolic consequence of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48:121–5.
Chowdhury TA, Escudier V. Poor glycaemic control caused by insulin induced lipohypertrophy. Br Med J. 2003;327:383–4.
Gentile S, Strollo F, Nefrocenter Research Study Group. Cost saving effects of a short-term educational intervention entailing lower hypoglycaemic event rates in people with type 1 diabetes and lipo-hypertrophy. Diabetes Res Clin Pract. 2018;143:320–1. https://doi.org/10.1016/j.diabres.2018.07.030.
Gentile S, Grassi G, Armentano V, et al. AMD-OSDI consensus on injection techniques for people with diabetes mellitus. Med Clin Rev. 2016;2(24):3. https://doi.org/10.21767/2471-299X.1000034.
Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–80. https://doi.org/10.2337/diacare.26.4.1176.
Strauss K, De Gols H, Hannet I, Partanen TM, Frid A. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diab Int. 2002;19:71–6. https://doi.org/10.1002/pdi.314.
Giorda CB, Ozzello A, Gentile S, HYPOS-1 Study Group of AMD. Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015;52:845–53. https://doi.org/10.1007/s00592-015-0713-4.
Giorda CB, Ozzello A, Gentile S, et al. Incidence and correlates of hypoglycemia in type 2 diabetes. The Hypos-1 study. J Diabetes Metab. 2014;5:344.
Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–76.
American Diabetes Association. Clinical practice recommendations 2008. Hypoglycaemia and employment/licensure. Diabetes Care. 2008;31(Suppl 1):S94–123.
American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Supp 1):S11–63. https://doi.org/10.2337/dc12-s011.
Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clin Proc. 2016;91:1212–23.
de Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 insulin injection technique questionnaire survey. J Diabetes. 2010;2:168–79. https://doi.org/10.1111/j.1753-0407.2010.00077.x.
International Diabetes Federation. Clinical guidelines taskforce. In: Global guideline for type 2 diabetes. 2012. http://www.idf.org/guideline-type-2-diabetes. Accessed 26 July 2016.
Gentile S, Guarino G, Guida P, Strollo F, On behalf of the AMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. SpringerPlus. 2016;5:563. https://doi.org/10.1186/s40064-016-1978-y.
Gentile S, Strollo F, Guarino G, et al. Factors hindering correct identification of unapparent lipohypertrophy. J Diabetes Metab Disord Control. 2016;3:00065. https://doi.org/10.15406/jdmdc.2016.03.00065.
Gentile S, Strollo F, Guarino G. Why are so huge differences reported in the occurrence rate of skin lipohypertrophy? Does it depend on method defects or on lack of interest? Diabetes Metab Syndr. 2019;13(1):682–6. https://doi.org/10.1016/j.dsx.2018.11.042.
Patil M, Sahoo J, Kamalathan S, et al. Assessment of insulin injection techniques among diabetes patients in a tertiary care centre. Diabetes Metab Syndr. 2017;11(Suppl 1):S53e6. https://doi.org/10.1016/j.dsx.2016.09.010.
Deng N, Zhang X, Zhao F, Wang Y, He H. Prevalence of lipohypertrophy in insulin-treated diabetes patients: a systematic review and meta-analysis. J Diabetes Investig. 2017. https://doi.org/10.1111/jdi.12742.
Gentile S, Strollo F, Della Corte T, Marino G, Guarino G. Insulin related lipodystrophic lesions and hypoglycemia: double standards? Diabetes Metab Syndr. 2018;12(5):813–8. https://doi.org/10.1016/j.dsx.2018.04.023.
Nakatani Y, Matsumura M, Monden T, Aso Y, Nakamoto T. Improvement of glycemic control by re-education in insulin injection technique in patients with diabetes mellitus. Adv Ther. 2013;30:897e906. https://doi.org/10.1007/s12325-013-0066-8.
Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):123–4. https://doi.org/10.1016/j.mayocp.2016.06.010.
Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: injecting complications and the role of the professional. Mayo Clin Proc. 2016;91(9):1224–30. https://doi.org/10.1016/j.mayocp.2016.06.012.
Acknowledgements
Special thanks are due to Paola Murano and to Members of AMD-OSDI Study Group on Injection Technique, for editorial assistance.
Funding
The paper was supported by a non-conditioning special grant for article processing charges from Nefrocenter Research Network, Naples, Italy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published: S. Gentile, F. Strollo, E. Satta, T. Della Corte, C. Romano, G. Guarino.
Authorship Contributions
SG and FS created the paper and wrote it. ES, TDC, GG, critically read the paper. All have complied with data collection, critically assessed the results, and approved the final text. All collaborators (see supplementary material) critically read and approved the final text.
Study Investigators
For full list of investigators see supplementary material.
Disclosures
S. Gentile, F. Strollo, E. Satta, T. Della Corte, C. Romano, and G. Guarino have nothing to declare.
Compliance with Ethics Guidelines
This study was conducted in conformance with good clinical practice standards. The study was led in accordance with the Declaration of Helsinki 1975, as revised in 2008, and was approved by Vanvitelli University, Naples, Italy and all the ethics committees of the centers participating in the study. (For full list of participating centers, see supplementary material.) Written informed consent was obtained from all participants before enrollment.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8223488.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gentile, S., Strollo, F., Satta, E. et al. Insulin-Related Lipohypertrophy in Hemodialyzed Diabetic People: a Multicenter Observational Study and a Methodological Approach. Diabetes Ther 10, 1423–1433 (2019). https://doi.org/10.1007/s13300-019-0650-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0650-2