Abstract
Introduction
OPTIMIZE evaluated the efficacy, safety and treatment satisfaction of insulin glargine 300 U/mL once daily (Gla-300 OD) in people with type 1 diabetes mellitus (T1DM) previously uncontrolled on basal insulin twice daily (BID) as part of basal–bolus therapy.
Methods
OPTIMIZE was a 28-week, prospective, interventional, single-arm phase 4 trial in adults with T1DM. At baseline, basal insulin BID treatment was switched to Gla-300 OD titrated to a fasting self-monitored blood glucose target of 4.4–7.2 mmol/L (80–130 mg/dL). The primary endpoint was the mean glycated haemoglobin (HbA1c) change from baseline to week 24. Secondary endpoints included self-monitored blood glucose, fasting-plasma glucose, hypoglycaemia and patient-reported outcomes including the Diabetes Treatment Satisfaction Questionnaire status version (DTSQs).
Results
Switching to Gla-300 OD significantly improved mean HbA1c (8.54% at baseline and 8.27% at week 24 [last observation carried forward, N = 94, p < 0.0001]; mean difference 0.27% [95% CI 0.15, 0.40]). There was a statistically significant decrease in fasting self-monitored blood glucose during the study (analysis of variance for repeated measures, p = 0.014; N = 72). Eight-point self-monitored blood glucose was significantly improved between baseline and week 24 for post-breakfast (p = 0.009), post-dinner (p = 0.009) and bedtime (p = 0.049) values. The study did not allow for any significant effects on confirmed and/or severe hypoglycaemia at the ≤ 3.9 mmol/L [≤ 70 mg/dL] or < 3.0 mmol/L [< 54 mg/dL] blood glucose cut-offs to be observed. Statistically significant improvements were observed in DTSQs total scores from baseline (24.1) to week 24 (29.4, p < 0.0001).
Conclusions
A basal–bolus regimen including Gla-300 OD was associated with improvements in HbA1c and treatment satisfaction in people with uncontrolled T1DM previously receiving basal–bolus insulin including a basal insulin BID analogue.
Trial Registration
EudraCT number: 2015-001186-46.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reducing the number of daily insulin injections for people with type 1 diabetes (T1DM) may help overcome a potential barrier to treatment adherence and persistence, as well as improve healthcare resource utilisation and costs |
The OPTIMIZE study evaluated efficacy, safety and patient satisfaction through treatment optimisation with once-daily insulin glargine 300 U/mL (Gla-300) in patients with T1DM, who were previously uncontrolled on twice-daily basal insulin |
It was hypothesised that switching from twice-daily basal insulin to once-daily Gla-300 as part of a basal–bolus regimen would lead to a reduction in HbA1c at week 24 |
A basal–bolus regimen including once-daily Gla-300 was associated with improvements in HbA1c and treatment satisfaction in people with uncontrolled T1DM previously receiving basal–bolus insulin including a twice-daily basal insulin analogue |
Introduction
The need for multiple daily insulin injections represents a burden of diabetes management and contributes to patient dissatisfaction with insulin therapy [1]. Reducing the number of daily injections may help overcome a potential barrier to treatment adherence and persistence, as well as improve healthcare resource utilisation and costs [1, 2]. In addition, reducing the burden of injections may improve quality of life and treatment satisfaction, which correlates with improvements in treatment flexibility and convenience [3].
The first-generation basal insulin analogues glargine 100 U/mL (Gla-100) and detemir (IDet) have longer durations of action at clinically relevant dose ranges with a single daily injection compared with neutral protamine Hagedorn (NPH) insulin [4,5,6]. Nevertheless, in some people with type 1 diabetes mellitus (T1DM), glycaemic control can only be improved with twice-daily (BID) dosing of Gla-100 and IDet [4, 7]. A single daily dose of the second-generation insulin glargine 300 U/mL (Gla-300) provides a longer duration of action (up to 36 h) and a more stable pharmacokinetic/pharmacodynamic profile than Gla-100 [8]. In people with long-duration T1DM, Gla-300 provided similar glucose control to Gla-100 once daily (OD), with a lower risk of hypoglycaemia in the first 8 weeks [9]. Moreover, in people with type 2 diabetes mellitus (T2DM) who switched from basal insulin BID to OD, Gla-300 provided similar glycaemic control with less hypoglycaemia compared with Gla-100 [10]. Switching from basal insulin BID to Gla-300 OD may provide similar benefits in T1DM. Albeit that people with T1DM typically use multiple daily insulin injections to control blood glucose levels [11], reducing the frequency of basal insulin injections for those who inject more than OD may help ease the burden of treatment. Therefore, the key objectives of the OPTIMIZE study were to evaluate efficacy, safety and patient satisfaction through treatment optimisation with Gla-300 OD in patients with T1DM, who were previously uncontrolled on basal insulin BID.
Methods
Study Design
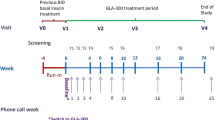
The OPTIMIZE study was a 28-week, multicentre, prospective, interventional, single-arm phase 4 trial conducted in Belgium and Canada (Supplementary Fig. 1). The study consisted of a 4-week run-in period, a 24-week Gla-300 treatment period, and a 2- to 7-day safety follow-up period. During the 4-week run-in period, patients continued their previous basal insulin BID regimen without any additional interventions and were specifically asked to record hypoglycaemic events. At baseline, patients were transferred from their previous basal insulin treatment to Gla-300 OD, while continuing their prandial insulin therapy for the duration of the study (see “Treatment” section). HbA1c was measured at the study site laboratory using a National Glycohemoglobin Standardization Program method at entry in run-in period (visit 0, week 4), baseline (visit 1, day 1), visit 2 (week 12) and visit 3 (week 24; final primary endpoint assessment visit). Telephone calls were scheduled at weeks 1, 2, 3, 4, 6, 8, 10, 16, 20 and during the week following the end of treatment (week 25), to assist with insulin titration, assess hypoglycaemia and any other adverse events (AEs), discuss concomitant medications and confirm compliance. The study was conducted in accordance with international ethical guidelines, including the Declaration of Helsinki, and the International Conference on Harmonisation guidelines for Good Clinical Practice, all applicable laws, rules, and regulations. Informed consent was obtained from all participants prior to the conduct of any study-related procedures.
Study Population
The key inclusion criteria were (1) people with T1DM aged ≥ 18 years and being treated BID with any basal insulin in combination with prandial rapid-acting insulin analogue for at least 1 year; and (2) HbA1c ≥ 8.0% (≥ 64 mmol/mol) and ≤ 10.0% (≤ 86 mmol/mol) at study entry. Key exclusion criteria were (1) treatment with glucagon-like peptide 1 (GLP-1) receptor agonists; and (2) use of systemic glucocorticoids (excluding topical application or inhaled forms) for 2 weeks or more within 8 weeks. Full inclusion and exclusion criteria are listed in Supplementary Table 1.
Treatment
At the end of the run-in period, the prior basal insulin was stopped and Gla-300 OD was self-administered in the morning by subcutaneous injection. The injection had to be administered at the same time each day between 06:00 and 10:00 and before breakfast. The Gla-300 dose was started on a unit-to-unit basis for those transferring from Gla-100 units/mL, unless the investigator thought it was necessary to reduce the dose to 80% of the prior Gla-100 dose for the patient’s safety. Those transferring from other basal insulins BID to Gla-300 OD were recommended to start at 80% of the previous total daily basal insulin dose. Gla-300 was titrated at least weekly, and preferably every 3 days, to a fasting self-monitored blood glucose (SMBG) of 4.4–7.2 mmol/L (80–130 mg/dL) [12]. If the median fasting SMBG was > 7.2 mmol/L (130 mg/dL) the dose was increased by 1 U (or more, at the discretion of the investigator). If the median fasting SMBG was between 4.4 and 7.2 mmol/L (80–130 mg/dL) the dose was maintained. If the median fasting SMBG was < 4.4 mmol/L (80 mg/dL) the dose was reduced by 1 U (or at the discretion of the investigator). In case of a single fasting SMBG measurement of < 3.9 mmol/L (< 70 mg/dL), it was recommended to decrease the Gla-300 dose by 1 U. Prandial insulin was continued and doses were adjusted at the discretion of the investigator and the patient.
Primary Endpoint
The primary endpoint was the mean HbA1c change from baseline to week 24.
Key Secondary Endpoints
Key secondary endpoints included mean HbA1c change from baseline to week 12 and mean fasting plasma glucose (FPG) change from baseline to weeks 12 and 24; change in fasting SMBG from baseline to weeks 4, 8, 12 and 24 and the change in 8-point SMBG from baseline to weeks 12 and 24; proportion of participants achieving HbA1c improvement from baseline to week 24 of at least 0.3% without documented (≤ 70 mg/dL or 3.9 mmol/L) and/or severe nocturnal hypoglycaemia (00:00–05:59) hypoglycaemia during the last 4 weeks of treatment.
Safety endpoints included confirmed (< 3.0 mmol/L [< 54 mg/dL] or ≤ 3.9 mmol/L [≤ 70 mg/dL]) and/or severe hypoglycaemia according to time (nocturnal [00:00–05:59] or any time of day) recorded during the run-in and the 24-week treatment period [13]. Other safety endpoints included AE, vital signs, body weight and change in basal insulin dose from baseline to the end of study.
OPTIMIZE also included patient-reported outcomes (PROs) such as change in Diabetes Treatment Satisfaction Questionnaire status version (DTSQs) scores, which measure treatment satisfaction and perceived frequency of hypoglycaemia and hyperglycaemia [14]. These were assessed between baseline and weeks 12 and 24. Patient satisfaction with the number of injections per day was assessed on a 7-point Likert scale (6 = very satisfied; 0 = very dissatisfied) at baseline and week 24. Mean change from baseline to week 24 in the Hypoglycaemia Fear Scale (HFS-II), which addresses behaviours and worries related to fear of hypoglycaemia in adults with T1DM, was documented for behaviour, worry and total scores [15,16,17]. AEs and serious AEs (SAEs) were recorded during the run-in, treatment and post-treatment follow-up period.
Data Analysis and Statistics
Primary and secondary efficacy endpoints were assessed in the intent-to-treat (ITT) population, which included all patients eligible for Gla-300 treatment at baseline, whatever the duration of their follow-up. A sample size of 90 evaluable patients was needed to evaluate the hypothesis of an HbA1c reduction of at least 0.3% between baseline and week 24 in a single-arm treatment group, assuming differences in standard deviation (SD) of 1.0%, with a 2-sided test at the 5% significant level, and a power of 80%. HbA1c change was assessed using paired t tests and the results were confirmed using univariate generalised linear model with baseline HbA1c values as a covariate (analysis of covariance). A hierarchical approach was used to evaluate the statistical significance of the secondary endpoints. If significance was demonstrated for the primary endpoint, then the secondary endpoints were tested in a predefined order and when an endpoint was not significant, no further testing was permitted. IBM SPSS Statistics (version 21.0 and eventual updates/upgrades) and StatXact (version 6.0 and eventual updates/upgrades) were used for the statistical analyses.
The safety population included all individuals exposed to at least one dose of Gla-300, regardless of the amount of treatment administered. The occurrence of hypoglycaemia during the last 4 weeks on treatment and during the 4-week run-in period was compared using the McNemar’s test. Changes in DTSQs total score and HFS-II scores were analysed using the same method as for the primary endpoint. Change in DTSQs sub-scores for hyperglycaemia and hypoglycaemia perception were analysed by Friedman’s test, and if significant, followed by Wilcoxon’s test. Change in patient satisfaction with the number of injections per day was analysed using a chi-square test.
Results
Patients
The study was conducted between 17 December 2015 and 10 May 2018 in 25 centres (14 in Belgium and 11 in Canada). In total, 58 (44.6%) and 72 (55.4%) participants were included from Belgium and Canada, respectively. There were 36 screen failures including HbA1c values outside the acceptable range (n = 26) with 24 (67%) having values < 8% (64 mmol/mol) and 2 (6%) having values > 10% (86 mmol/mol); clinically significant abnormality or major systemic disease (n = 1; 3%); withdrawal of consent (n = 3, 8%); not on insulin BID at study entry (n = 1, 3%); blood taken outside of the allowed time window and not fasting (n = 1, 3%); not considered eligible anymore at the baseline visit (visit 1) after the run-in period (n = 4, 11%). Overall, 94 patients (44 men and 50 women; 48 in Belgium and 46 in Canada) were eligible at screening (visit 0), completed the 4-week run-in period and were still eligible at baseline (visit 1). These participants constituted the evaluable, the ITT and the safety populations (Supplementary Fig. 2). The response rate to scheduled telephone calls was high, 91 participants at week 12 responded and 92 participants at week 24; the highest number of missed responses was 8 at week 20. The mean (± SD) duration of exposure to study medication was 24.0 ± 3.3 weeks.
Baseline Demographics
The ITT and safety populations comprised 94 patients (53.2% women) with a mean (SD; range) age of 49.4 (13.1; 23–79) years and an average T1DM duration of 27 years (Table 1). At screening, 45.7% of patients were receiving Gla-100, 52.1% IDet and 2.1% NPH insulin. Microvascular complications were common, with almost 50% of participants having retinopathy (Table 1).
Change in HbA1c
There was a reduction in HbA1c from 8.70% (72 mmol/mol) to 8.54% (69 mmol/mol) during the run-in period. Mean (SD) HbA1c then decreased significantly from 8.54% (0.56) (69 mmol/mol) at baseline to 8.27% (0.62) (67 mmol/mol) at week 24 (last observation carried forward, N = 94, p < 0.0001); mean difference [95% confidence interval [CI] 0.27% [0.15, 0.40] [3.3 mmol/mol]) (Table 2, Fig. 1). A significant decrease in HbA1c was observed between baseline and week 24 in participants taking insulin glargine at baseline (p = 0.031; mean difference [95% CI] 0.20 [0.02, 0.38]) and the gain in efficacy was greater in those taking IDet at baseline (p < 0.0001; mean difference [95% CI] 0.38 [0.19, 0.56]). The reduction in HbA1c was similar in the subgroups of participants from Belgium and Canada (p = 0.077). HbA1c improved significantly from baseline to week 12 (reduction from 8.54% [69 mmol/mol] to 8.14% [65 mmol/mol], p < 0.0001). HbA1c values at all time points are included in Fig. 1.
A total of 48 out of 92 participants (52.2%, 95% CI 41.5–62.7%) achieved an HbA1c improvement from baseline to week 24 of ≥ 0.3%. Of these participants, 5 (5.4%, 95% CI 1.8–12.2%) achieved this HbA1c improvement without nocturnal confirmed (≤ 3.9 mmol/L or ≤ 70 mg/dL) and/or severe hypoglycaemia during the last 4 weeks of treatment.
Change in Other Glucose Measures
There was a statistically significant decrease in fasting SMBG during the study (analysis of variance for repeated measures, p = 0.014; N = 72). Decreases in fasting SMBG were statistically significant between baseline and the telephone calls at week 8 (p = 0.001) and week 12 (p = 0.019) but the difference between baseline and week 24 was not significant (p = 0.106; paired t test; Fig. 2a). For the 8-point SMBG scale, there were statistically significant improvements between baseline and week 24 for post-breakfast (p = 0.009), post-dinner (p = 0.009) and bedtime (p = 0.049) SMBG (Fig. 2b). The changes in 8-point SMBG scale from baseline to week 24 with Gla-100 or IDet as previous basal insulins BID are shown in Fig. 3a, b. There was a non-significant trend towards improvement in FPG ± SD from baseline (217.8 ± 92.0 mg/dL [12.1 ± 5.1 mmol/L]) to month 6 (203.9 ± 77.7 mg/dL [11.3 ± 4.3 mmol/L]; p = 0.05).
Hypoglycaemic Events
OPTIMIZE did not reveal any significant differences in total, nocturnal, severe, symptomatic and confirmed hypoglycaemic event rates between the run-in period and the last 4 weeks of treatment (Supplementary Table 2).
Safety
Overall, there were 248 AEs (other than hypoglycaemic events) in 71 (75.5%) patients during the study period (40 during the pretreatment period, 203 during the treatment period and two during the post-treatment period). During the treatment period, 10 AEs in 7 (7.4%) participants were considered related to Gla-300: epilepsy (n = 1), hypoglycaemia (n = 4), weight increased (n = 1), injection site hyperaesthesia (n = 1), paraesthesia (n = 1), fungal infection (n = 1) and peripheral oedema (n = 1). Four SAEs were reported during the treatment period: hypoglycaemia (n = 1), uterine leiomyoma (n = 1), thrombosis (n = 1) and hypoglycaemic coma (n = 1). Except for the hypoglycaemia event, which was considered related to Gla-300, the other SAEs were not considered related to treatment.
Insulin Dose and Body Weight
There was a statistically significant (p = 0.03) change in the total basal insulin dose (mean [SD]) from baseline (37.4 [21.9] U), which reflects the mean total daily dose of the previous basal insulin BID over three measurements in the week prior to the switch to Gla-300, to the dose of Gla-300 at the end of the study (39.8 [22.0] U; Supplementary Fig. 3A). There was no statistically significant (p = 0.20) modification of the total prandial insulin dose over time (32.2 [19.1] U at baseline vs 27.0 [15.6] U at last telephone call; N = 71; Supplementary Fig. 3A). There was no statistically significant change in mean ± SD body weight between baseline (81.3 ± 17.7 kg), week 12 (81.7 ± 18.0 kg) and week 24 (82.0 ± 18.1 kg, p = 0.11; Supplementary Fig. 3B).
Patient-Reported Outcomes
Statistically significant improvements were observed in DTSQs total treatment satisfaction scores from baseline to week 24 (Fig. 4). There was a statistically significant improvement in perceived hyperglycaemia scores when comparing baseline, week 12 and week 24 visits (Supplementary Table 3). Patients’ satisfaction scores concerning the number of injections improved significantly (p < 0.0001) from baseline to week 24 (Supplementary Table 4). Compared with baseline, there was no significant increase in HFS-II total scores, or scores for worry and behaviour, from baseline to week 24 (Table 3).
Discussion
The results of OPTIMIZE indicate that in people with T1DM previously treated with basal insulin BID in combination with prandial rapid-acting insulin, switching to Gla-300 OD provided a significant reduction in HbA1c and improvements in treatment satisfaction. A significant reduction in HbA1c was observed irrespective of whether participants were on prior Gla-100 BID or IDet BID, although the greatest reduction was observed when the participants were switched from IDet BID to Gla-300 OD. The statistically significant improvement in HbA1c, while modest, was achieved with an increase in overall insulin dose but a (non-significant) decrease in prandial insulin doses. This may suggest that the 24-week duration of OPTIMIZE was not sufficient to allow proper adjustment of the prandial insulin in the context of the longer-acting basal insulin.
It is important to note that the OPTIMIZE population had a long history of T1DM. Almost half of the population had microvascular complications that are reflective of a long duration of T1DM and poor control. The OPTIMIZE population had a mean HbA1c > 8% (> 64 mmol/mol) despite administering basal insulin BID, which is a clinical profile that would often prompt healthcare professionals to consider the option of insulin pump therapy. Although the reason for not progressing to insulin pump technology is not known, the fact that these patients remained on multiple daily insulin injections may indicate that they are less able (e.g. through lack of availability or insurance coverage) or unwilling to adopt new treatment approaches, and therefore reflect a more “difficult-to-treat” population. Hence, while this population is representative of people with T1DM in the real world, they are more challenging to treat than typical T1DM populations in many randomised controlled trials. For example, in EDITION 4 participants had a mean age of 47 years, had been diagnosed for 21 years and 15% were taking basal insulin BID [9], whereas the OPTIMIZE group was on average aged 49 years and had been living with T1DM for approximately 27 years and all were on basal insulin BID. This long duration of disease was reflected by high baseline HbA1c (8.5%, 69.4 mmol/L) and FPG (217.8 mg/dL [12.1 mmol/L]). The OPTIMIZE population had a mean body mass index in the overweight range, highlighting the difficulty of weight management for individuals with T1DM. Together, these data suggest that this was a difficult-to-treat population with long-term T1DM that had been poorly controlled despite basal insulin BID.
The reduction in HbA1c from baseline was not accompanied by an increased rate of hypoglycaemic events. This may reflect the longer duration of action and more stable pharmacokinetic/pharmacodynamic properties of Gla-300 versus the first-generation basal insulins used BID before the study [4, 8]. In OPTIMIZE, participants self-recorded hypoglycaemic events in study diaries throughout the run-in and study periods, and there was evidence that these had not been completed in some cases. Therefore, it is possible that some hypoglycaemic events could have been misrecorded or overlooked. In OPTIMIZE, no participants achieved the typical target HbA1c of < 7% (53 mmol/mol) that is recommended by the American Diabetes Association for most non-pregnant individuals with T1DM [18]. This finding may indicate that even in the trial setting, suboptimal insulin titration is an issue. Indeed, we saw that the total basal insulin dose increased but bolus insulin dose decreased (although this was not significant). This would suggest that at week 24 prandial insulin doses were still not optimised, and that the study period was probably insufficient to properly adjust the prandial insulin in the context of the new long-acting Gla-300. Failure to appropriately titrate insulin is common in the real-world setting, where fear of hypoglycaemia, infrequent physician visits and/or lack of diabetes expertise on the part of the healthcare professional may prevent timely dose increases [19]. Additionally, in a difficult-to-treat population such as that included in OPTIMIZE, other issues including failure to optimise the prandial insulin dose and poor compliance with dietary recommendations may impact outcomes and, indeed, the typical targets may not be appropriate for such individuals. Alternatively, the failure to achieve HbA1c targets may indicate that the study duration was too short to adequately titrate the new basal insulin and bolus insulin dosing.
Switching to Gla-300 OD was associated with significant improvements in the PROs for total and hyperglycaemia perception DTSQs scores (Fig. 4), and for patient satisfaction relating to number of injections. This is consistent with previous reports that patient satisfaction and quality of life are related to flexible, convenient insulin dosing regimens [2, 3]. The improvement in hyperglycaemia perception DTSQ scores may reflect the 8-point SMBG profile, which demonstrates lower fluctuations in blood glucose concentrations with Gla-300 OD and reduced peaks of blood glucose post-breakfast and post-dinner compared with basal insulin BID. Given the lower blood glucose peak post-breakfast with Gla-300 OD at 6 months versus baseline, there was a smaller drop in blood glucose levels post-breakfast to pre-lunch, which may have contributed to the hyperglycaemia perception DTSQs scores. There is limited evidence of improvement in treatment satisfaction being associated with improved feelings of self-efficacy and better treatment adherence [20]. Certainly, there appears to be a link between higher treatment satisfaction and lower treatment dropout [20]. While the 24-week follow-up in OPTIMIZE is short for assessing treatment compliance, a low level of dropout was observed in this study (one discontinuation and one for minor protocol deviations). No significant improvements in hypoglycaemia perception DTSQs score and HFS-II score were observed, which corresponds with the finding that there was no significant change in the number of hypoglycaemia events during the study.
The main weaknesses of OPTIMIZE are inherent to a real-world study design, notably the lack of a control group and the unblinding of staff and participants. Given the lack of a control group we cannot say for certain what the outcome would have been if participants had remained on basal insulin BID, so the observed improvements in HbA1c following the switch to Gla-300 OD should be considered as an indicative result that should be confirmed in a controlled trial setting. The open-label design may positively bias outcomes if participants perceive that they are being switched to a “newer” or “more advanced” treatment. However, the switch to a new and unfamiliar basal insulin may worry individuals and it may take time for them to become more comfortable with the switch. Hence, such positive and negative perceptions could potentially affect the more subjective PRO scores. The main strength of OPTIMIZE is that it includes a population that is challenging to treat with a long history of poor glycaemic control, so it is possible that PRO benefits from Gla-300 are more obvious in this population; however, this hypothesis would need to be confirmed in a randomised controlled trial.
Further studies in a larger population of people with T1DM in real-world practice are required to determine whether the benefits provided by switching from basal insulin BID to Gla-300 OD are associated with improvements in treatment.
Conclusions
In patients with uncontrolled T1DM and treated with a basal–bolus schema, switching from a basal insulin BID analogue to Gla-300 OD was associated with statistically significant improvements in HbA1c by week 24 with no increase in the number of hypoglycaemic events and with an improved overall treatment satisfaction.
References
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–9.
Rathmann W, Dippel FW, Kostev K. Different injection frequencies of basal insulins in type 2 diabetes patients under real-life conditions: a retrospective database analysis. J Diabetes Sci Technol. 2013;7(5):1354–8.
Ashwell SG, Bradley C, Stephens JW, Witthaus E, Home PD. Treatment satisfaction and quality of life with insulin glargine plus insulin lispro compared with NPH insulin plus unmodified human insulin in individuals with type 1 diabetes. Diabetes Care. 2008;31(6):1112–7.
Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19(1):3–12.
Rosenstock J, Park G, Zimmerman J, US Insulin Glargine Type 1 Diabetes Investigator Group. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. US Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care. 2000;23(8):1137–42.
Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107–12.
Albright ES, Desmond R, Bell DS. Efficacy of conversion from bedtime NPH insulin injection to once- or twice-daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy. Diabetes Care. 2004;27(2):632–3.
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units/mL provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units/mL. Diabetes Care. 2015;38(4):637–43.
Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015;38(12):2217–25.
Roussel R, Emden MC, Fisher M, et al. Glycaemic control and hypoglycaemia in people with type 2 diabetes switching from twice-daily basal insulin to once-daily insulin glargine 300 U/mL or insulin glargine 100 U/mL (EDITION 1 and EDITION 2 subgroup analysis). Diabetes Obes Metab. 2018;20(2):448–52.
Laubner K, Molz K, Kerner W, et al. Daily insulin doses and injection frequencies of neutral protamine hagedorn (NPH) insulin, insulin detemir and insulin glargine in type 1 and type 2 diabetes: a multicenter analysis of 51 964 patients from the German/Austrian DPV-wiss database. Diabetes Metab Res Rev. 2014;30(5):395–404.
American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97–111.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. In: Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Hove: Harwood; 1994. p. 111–132.
Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801–6.
Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–21.
Irvine A, Cox D, Gonder-Frederick L. The fear of hypoglycemia scale. In: Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Hove: Harwood; 1994. p.133–158.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–70.
Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14(8):675–82.
Saisho Y. Use of Diabetes Treatment Satisfaction Questionnaire in diabetes care: importance of patient-reported outcomes. Int J Environ Res Public Health. 2018;15(5):947.
Acknowledgements
We thank the participants of this study.
B-Switch Group: Katrien Benhalima (UZ Leuven, Leuven, Belgium); Veronique Buyse (O.L.V Lourdes Ziekenhuis, Waregem, Belgium); Ides Michel Colin (CHR Mons-Hainaut, Mons, Belgium); Martin D’Amours (Clinique des Maladies Lipidiques de Québec, Quebec, Canada); Christophe De Block (UZ Antwerpen, Edegem, Belgium); Emilie Debue (CH de Mouscron, Mouscron, Belgium); Richard Dumas (Centre de recherche clinique de Laval, Quebec, Canada); Thomas Elliott (BC Diabetes, Vancouver, Canada); Philippe Ernest (CHU De Liège-Sart Tilman, Liège, Belgium); Vincent Ers (Cliniques du Sud Luxembourg, Arlon, Belgium); Pieter Gillard (UZ Leuven, Leuven, Belgium); Paul Guffens (St. Nikolaus-Hospital Eupen, Eupen, Belgium); Sam Henein (SKDS Research Inc, Newmarket, Canada); Michel Hermans (Cliniques Universitaires Saint-Luc, Brussels, Belgium); Irene Hramiak (St. Joseph’s Health Care London, London, Canada); Wen Hu (LMC Clinical Research Inc., Montreal, Canada); Carmen Hurd (Health Sciences Centre Winnipeg, Winnipeg, Canada); Bernard Jandrain (CHU De Liège-Sart Tilman, Liège, Belgium); Esther Jousten (CHR de la Citadelle, Liège, Belgium); Norman Kalyniuk (SKDS Research Inc, Newmarket, Canada); Anna Lam (University of Alberta, Edmonton, Canada); Toon Maes (Imelda Ziekenhuis, Bonheiden, Belgium); Tammy McNab (University of Alberta, Edmonton, Canada); Katia Mekeirele (O.L.V Lourdes Ziekenhuis, Waregem, Belgium); Laurie Mereu (University of Alberta, Edmonton, Canada); Hortensia Mircescu (Institut de recherches cliniques de Montréal, Montreal, Canada); Daniel Nicolaij (AZ Groeninge, Kortrijk, Belgium); Frank Nobels (Onze-Lieve-Vrouw-Ziekenhuis, Aalst, Belgium); Philippe Oriot (CH de Mouscron, Mouscron, Belgium); Terri Paul (St. Joseph’s Health Care London, London, Canada); Françoise Pirard (CHR Mons-Hainaut, Mons, Belgium); Vanessa Preumont (Cliniques Universitaires Saint-Luc, Brussels, Belgium); Remi Rabasa-Lhoret (Institut de recherches cliniques de Montréal, Montreal, Canada); Waheed Rehman (LMC Clinical Research Inc., Ville St-Laurent, Canada); André Scheen (CHU De Liège-Sart Tilman, Liège, Belgium); Peter Senior (University of Alberta, Edmonton, Canada); Tamara Spaic (St. Joseph’s Health Care London, London, Canada); Sara Stafford (BC Diabetes, Vancouver, Canada;) Youri Taes (AZ Sint-Jan Brugge, Brugge, Belgium); Vanessa Tardio (AMIR, Montreal, Canada); Vincianne Thielen (CHR de la Citadelle, Liège, Belgium); George Orestis Tsoukas (AMIR, Montreal, Canada); Michael Tsoukas (AMIR, Montreal, Canada); Paul Van Crombrugge (Onze-Lieve-Vrouw-Ziekenhuis, Aalst, Belgium); Annick Van den Bruel (AZ Sint-Jan Brugge, Brugge, Belgium); Luc Van Gaal (UZ Antwerpen, Edegem, Belgium); Gerd Vanhaverbeke (AZ Groeninge, Kortrijk, Belgium); Sylva van Imschoot (AZ Sint-Jan Brugge, Brugge, Belgium); Chris Vercammen (Imelda Ziekenhuis, Bonheiden, Belgium); Eric Weber (Cliniques du Sud Luxembourg, Arlon, Belgium); Vincent Woo (Health Sciences Centre Winnipeg, Winnipeg, Canada); Jean-Francois Yale (LMC Clinical Research Inc., Montreal, Canada); Zeina Yared (LMC Clinical Research Inc., Ville St-Laurent, Canada).
Funding
The work and funding of the journal’s Rapid Service fees was supported by Sanofi.
Medical Writing and/or Editorial Assistance
Medical writing support provided by Vicky Hinstridge, BPharm, of Fishawack Communications Ltd, was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Chantal Mathieu acts as author on behalf of the B-Switch Group.
Disclosures
Chantal Mathieu serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Dianax and UCB. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for CM from Medtronic, Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Roche, Abbott, ActoBio Therapeutics and Novartis; Chantal Mathieu serves or has served on the speaker bureau for Novo Nordisk, Sanofi, Merck Sharp and Dohme, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. S. John Weisnagel has received financial research support from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Novartis, Novo Nordisk, Sanofi-Aventis, and has received honoraria for conferences or advisory meetings from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Omnipod, and Sanofi-Aventis. Jacques Bruhwyler was an external Contractor for Data Management, Biostatistics and Medical Writing. Kathy Alexandre and Peter Stella are Sanofi employees.
Compliance with Ethics Guidelines
The study was conducted in accordance with international ethical guidelines, including the Declaration of Helsinki, and the International Conference on Harmonisation guidelines for Good Clinical Practice, all applicable laws, rules, and regulations. Informed consent was obtained from all participants prior to the conduct of any study-related procedures.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
The members of “B-Switch Group” are listed in Acknowledgements section.
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11342231.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mathieu, C., Weisnagel, S.J., Stella, P. et al. Impact of Switching from Twice-Daily Basal Insulin to Once-Daily Insulin Glargine 300 U/mL in People with Type 1 Diabetes on Basal–Bolus Insulin: Phase 4 OPTIMIZE Study. Diabetes Ther 11, 495–507 (2020). https://doi.org/10.1007/s13300-019-00749-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00749-1