Abstract
Introduction
This study aimed to determine, in close to real-life conditions, the efficacy and safety of switching from any basal insulin to insulin glargine 300 U/mL (Gla-300) in patients with uncontrolled type 2 diabetes (T2D).
Methods
This was an interventional, multicenter, single-arm, prospective study with a 24-week treatment phase. Adult patients with T2D treated with basal insulin with or without other antidiabetics, HbA1c > 7.5%, and fasting self-monitored blood glucose (F-SMBG) > 130 mg/dL (mean of three measures) at baseline were included. Insulin dose was titrated to reach F-SMBG 90–130 mg/dL. Efficacy and safety were assessed at 12 weeks (W12) and 24 weeks (W24). The main outcome parameter was HbA1c change between baseline and W24. Safety parameters included self-reported hypoglycemia (any type). Patients’ satisfaction with the treatment was assessed by the Diabetes Treatment Satisfaction Questionnaire (DTSQ).
Results
A total of 140 patients were included and 137 were treated. Mean HbA1c decreased from 8.64% at baseline to 8.14% at W12 (mean difference [95% CI] − 0.51% [− 0.64; − 0.38]) and 8.01% at W24 (− 0.64% [− 0.81; − 0.46]). Target F-SMBG was reached in 35.0% of the patients at W12 and 38.4% at W24. The percentages of patients reaching HbA1c levels < 7.0%, < 7.5%, and < 8.0% at W24 were 11.4%, 29.5%, and 50.8%, respectively, while only 31.6% had an HbA1c value < 8.0% at baseline. HbA1c reduction was greater in patients with higher baseline levels. During the treatment phase, 46.0% of the participants had at least one hypoglycemia event; 31.4% documented symptomatic hypoglycemia, 2.2% severe hypoglycemia, and 12.2% nocturnal hypoglycemia. Treatment satisfaction increased by 20% between baseline and W24.
Conclusion
These data, derived from close to real-life practice in France, confirm the reassuring results of randomized trials on the efficacy and safety of Gla-300.
Trial Registration
EudraCT number 2015-002416-33.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Type 2 diabetes (T2D) is a frequent disease with a very heavy human and economic burden. |
New-generation long-acting insulin analogues have been launched in the past few years and randomized controlled trials have shown improved glucose control with less hypoglycemia with those new formulations in patients with T2D. |
The efficacy and safety in a real-life setting of switching from any basal insulin to insulin glargine 300 U/mL (Gla-300) have not been studied yet. |
What was learned from the study? |
This real-life study confirms the efficacy and safety of Gla-300 that was shown in randomized controlled trials. |
Gla-300 appears in close to real-life conditions as an efficient and safe alternative in patients with uncontrolled T2D on basal insulin. |
Introduction
In France, in 2017, about 3.28 million individuals aged 20–79 years, i.e., 7.3% of this age range, were living with diabetes and this disease was responsible for more than 18,000 deaths [1]. Among patients with diabetes, more than 90% have type 2 diabetes (T2D). It has been extensively shown that cardiovascular outcomes and mortality worsen with higher glycated hemoglobin (HbA1c) value [2] with a legacy effect of early intensive glucose control [3] even if HbA1c target should be personalized [4]. Although new therapeutic options have been available, insulin therapy is indicated as early as in second or third line [5] and is used in about 20% of French patients with T2D [6]. It has been shown that early initiation of basal insulin therapy with insulin glargine 100 U/mL (Gla-100) in T2D is safe [7] in terms of cardiovascular outcomes and it has been associated with better glucose control 4 years later [8]. More recently, a new formulation of insulin glargine that delivers the same amount of insulin as Gla-100 in one-third of the volume, insulin glargine 300 U/mL (Gla-300), was launched (in June 2016 in France). It was shown that Gla-300, owing to a smoother pharmacokinetics/pharmacodynamics (PK/PD) profile and longer duration of action vs Gla-100, improved glucose control with less 24-h and nocturnal hypoglycemia compared to Gla-100 in a broad population of patients with T2D [9]. However, those were randomized controlled trials (RCTs) with treat-to-target design and they included few participants from Europe and especially from France. Therefore, they were not representative of real-word clinical conditions and daily clinical practice in France. The TRANSITION 2 study was designed to determine, in close to real-life conditions, if the switch from any basal insulin to Gla-300, in a large population of patients using an insulin titration algorithm, resulted in the same benefits.
Methods
Study Design
TRANSITION 2 was an interventional, phase IV, multicenter, prospective, open, single-arm 24-week study designed to describe the efficacy and safety of Gla-300 in patients with suboptimal glucose control on basal insulin with or without other antidiabetics for whom a change in basal insulin was indicated according to the investigator. The primary efficacy outcome was the variation of HbA1c level at 24 weeks (W24). Secondary objectives included the description of Gla-300 effects on fasting blood glucose value, insulin dose, weight variation, as well as the effects on the rate and incidence of hypoglycemia, the treatment tolerance, and the patient satisfaction. The expected effects of treatment with Gla-300 according to the reasons for change in basal insulin and the adherence to the titration algorithm were also analyzed.
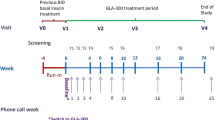
The study design is shown in Fig. 1.
After the screening visit (V0), patients entered into a 4-week run-in phase with their usual treatment in order to validate their eligibility in terms of HbA1c level and fasting self-monitored blood glucose (F-SMBG) at the inclusion visit (V1). Patients who were still eligible could enter into the 24-week treatment phase: basal insulin was switched to Gla-300. The non-insulin medications were continued unchanged provided they had been taken at stable dose for at least 8 weeks before V0. The type and dose of those treatments could not be changed during the study unless for safety reasons leading to dose lowering or drug discontinuation. Patients were encouraged to follow their usual diet and physical activity pattern throughout the study, according to the individualized advice previously provided. An intermediate visit was scheduled at W12 (V2) and the final visit was at W24 (V3). The treatment phase between V1 and V2 was the main titration phase although changes could be done thereafter. Moreover, nine phone calls were scheduled: each week during the first month, then every other week up to V2, at W15 and at W18.
Inclusion and Exclusion Criteria
Individuals at least 18 years old were eligible to enter the study if they had T2D treated with basal insulin (one or two daily injections) for at least 6 months at the screening visit and if a change in basal insulin was indicated according to the investigator because of suboptimal glucose control, titration difficulties, hypoglycemia in the previous 6 months, or fear of hypoglycemia.
Inclusion criteria required an HbA1c value > 7.5% (measured during the week before V1) and a mean result of the last three F-SMBG values during the week preceding V1 (or at least two out of the three last values) > 130 mg/dL. Basal insulin dose had to be stable (± 20%) in the previous 8 weeks as well as non-insulin treatment.
Patients could not be included if they were pregnant or planning a pregnancy, if their daily insulin requirements were greater than 1.2 unit/kg, if they were using prandial insulin, or if they had a disease or a treatment that could interfere with insulin requirements and/or HbA1c dosage. All participants provided written informed consent and the study was conducted in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the revised Declaration of Helsinki, 1964 (ethical agreement number 1-15-22, CPP Sud-Ouest et Outre-Mer 1).
Treatment Changes
Gla-300 was administered once daily using a pre-filled insulin pen (Toujeo® Solostar®) in the evening, from dinnertime to bedtime according to the patient’s and the investigator’s choice at the same time of the day ± 3 h if needed. The time of injection could differ from the time of injection of the previous basal insulin. Pens were labeled for the study. Initial dose was equal to the dose with the previous basal insulin when this one was injected once daily (80% of the total insulin dose if twice daily). Gla-300 dose was titrated at least weekly, or every 3–4 days when possible, according to the titration algorithm (Table 1). Non-insulin treatment remained unchanged during the whole study unless a treatment had to be stopped for safety reason according to the investigator. Global treatment adherence was assessed by the investigator between V1 and V2 and between V1 and V3 as ≥ 80% or < 80% according to the remaining insulin in the returned pens, whereas the adherence to the titration algorithm was evaluated at V2 and V3 according to the patient’s logbook (F-SMBG, insulin dose, hypoglycemia events, etc.).
Outcome Parameters
The primary efficacy outcome parameter was the mean change in HbA1c value between V1 and V3. Secondary efficacy parameters included the percentage of patients reaching the F-SMBG target range (90–130 mg/dL) or the American Diabetes Association (ADA)-recommended F-SMBG target range (80–130 mg/dL) [10] at V2 and V3, the percentage of patients reaching an HbA1c value < 7.0%, < 7.5%, or < 8.0% at V3, the mean reduction in HbA1c between V1 and V3 across the pre-defined baseline HbA1c subgroups (≤ 8.0%, 8.0–9.0%, > 9.0%), and those reaching a ≥ 0.3% reduction in HbA1c, the mean change in fasting blood glucose value between V1 and V2 or V3.
Safety outcome parameters included the incidence and rate of hypoglycemia events, the report of adverse events and serious adverse events, weight changes, and Gla-300 dose during the 24-week treatment phase. Hypoglycemia was considered as symptomatic documented if hypoglycemia symptoms were present with SMBG value ≤ 70 mg/dL or ≤ 54 mg/dL. Nocturnal hypoglycemia was defined as occurring between 00:00 and 05:59 and severe hypoglycemia required assistance. Treatment emergent adverse events occurred between the first injection of Gla-300 and 2 days after the last injection.
The investigator assessed the patient’s adherence to the insulin titration algorithm at visits V2 and V3 based on the data (F-SMBG, insulin doses, and other events such as hypoglycemia) noted by the patient in his/her logbook. Investigators had to determine whether adherence was low (< 50%), moderate (50–75%), or good (> 75%).
Patients’ satisfaction with the change of treatment was assessed using the change in the Diabetes Treatment Satisfaction Questionnaire (DTSQ) scores between V1 and V3. Investigators were asked for each participant at V2 and V3 if they observed the anticipated impact of the treatment change according to the reason they had for basal insulin switch.
Statistics
Initially 350 patients were to be included; assuming 10% of patients were not evaluable at 24 weeks, the precision (half-length of 95% CI) to describe the mean change in HbA1c was estimated to 0.13%, with an estimated standard deviation (SD) of 1.2%. Finally, the SD was 0.9, so with 136 assessed patients, an acceptable precision of 0.16% was observed. The change in HbA1c between V1 and V3 is described in terms of mean difference, with a 95% confidence interval (CI) on the modified intention to treat (mITT) population (patients enrolled in the study who received at least one dose of treatment and who could be evaluated for the primary endpoint, had a baseline HbA1c measurement, and at least one measurement after the first administration of treatment). Sensitivity analyses were done on the per protocol (PP) population (without any major deviation from the study protocol) and completers of mITT population (patients who completed the study and did not interrupt study treatment). The analyses of secondary efficacy evaluation endpoints and safety are descriptive.
Results
Study Population
Among the 194 screened subjects, 140 patients were included between January 2016 and January 2017 in 51 centers. The study flow chart is shown in Fig. 2. The safety population included 137 patients, the mITT population 136 patients, and the PP population 104 patients (Supplementary Table S1). The most frequent microvascular complication was nephropathy (27.2%) (Table 2) and 27.2% had a history of cardiovascular disease (myocardial infarction in 3.7%, coronary heart disease in 6.6%, atrial fibrillation in 3.7%).
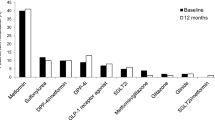
Basal insulin at V1 was Gla-100 (67.9%) or insulin detemir (32.1%). Besides insulin, patients were treated mainly with other antidiabetic treatments (Table 3) including glucagon-like peptide 1 receptor agonist (GLP-1RA) in 67 patients (49.3%). Among them, two patients started the treatment after V1, whereas five patients stopped the treatment at V1.
The adherence to treatment evaluated by the investigator at V2 and V3 was more than 80% in respectively 94.8% and 97.7% of the patients. The adherence to titration algorithm was considered by the investigator as good (> 75%) in 70.4% and 74.2% of the patients at V2 and V3, respectively; it was moderate (50–75%) in 19.3% and 19.7%, respectively.
Primary Efficacy Parameter
The mean HbA1c [95% CI] in the mITT population decreased from 8.64% [8.50; 8.79] at V1 to 8.14% [7.98; 8.30] at V2 (mean difference − 0.51% [− 0.64; − 0.38]) and 8.01% [7.84; 8.17] at V3 (− 0.64% [− 0.81; − 0.46]) (Fig. 3). Results were comparable when calculated on the PP population (n = 104) with mean HbA1c differences of − 0.52% [− 0.67; − 0.37] and − 0.68% [− 0.88; − 0.49] versus baseline at V2 and V3, respectively.
Secondary Efficacy Parameters
When considering the study F-SMBG target (90–130 mg/dL), the percentage of patients who reached this range (mean of the last three tests) was 35.0% at V2 and 38.4% at V3 (171.6 ± 40.9 mg/dL at V1). If the target range was extended to the ADA target (80–130 mg/dL), the percentages of patients reaching the target were respectively 39.0% and 42.4%.
When considering the patients who reached an HbA1c value < 7.0%, < 7.5%, and < 8.0%, it was observed that 5.9%, 23.5%, and 47.8% of the subjects at V2 and 11.4%, 29.5%, and 50.8% at V3, respectively, could reach the targets while only 31.6% had an HbA1c value < 8.0% at V1.
The percentage of patients with a ≥ 0.3% reduction in HbA1c was 65.4% at V2 and 72.0% at V3 (68.3% at V3 in those also treated with a GLP-1RA versus 75.0% in those without GLP-1RA). Among patients with a baseline HbA1c level ≤ 8.0% (n = 43), 8.0–9.0% (n = 57), and > 9.0% (n = 36), the percentages of patients reaching an HbA1c reduction ≥ 0.3% were respectively 51.2%, 64.9%, and 83.3% at V2 and 95.3%, 98.2%, and 97.2% at V3.
The main reason for switching insulin was an elevated HbA1c (94.1%) followed by titration difficulties (39.7%). Fear of hypoglycemia and hypoglycemia frequency were much less frequent reasons (respectively 8.1% and 5.1%). Multivariate logistic regression analysis showed that an elevated HbA1c as the main reason for changing basal insulin was the main parameter associated with HbA1c reduction ≥ 0.3%.
Safety Parameters
A total of 122 out of 137 patients (89.1%) had at least 23 weeks of treatment with Gla-300 and mean duration of treatment was 23.5 ± 3.6 weeks. Mean basal insulin dose increased from 46.1 ± 20.9 U/day at inclusion to 57.1 ± 28.7 U/day at V2 and 57.8 ± 27.7 U/day at V3 after insulin titration (median value 0.49 ± 0.21, 0.61 ± 0.27, and 0.61 ± 0.27 U/kg/day, respectively). During the treatment phase, at least one adverse event occurred in 56.9% of the patients (176 events) including 14 serious adverse events in 13 patients (9.5%) with no death but five treatment discontinuations (3.6%). None of the adverse events was considered by the investigators as being related to Gla-300. The main serious adverse event was poor glycemic control (n = 4; 2.9%).
Weight and blood pressure values remained stable throughout the study. Body mass index was 32.6 ± 5.3 kg/m2 at baseline, 32.8 ± 5.3 kg/m2 at V2, and 32.6 ± 5.3 kg/m2 at V3.
Hypoglycemia rate increased from 1.66 per patient-year during the screening phase to 5.15 during the treatment phase and 7.83 during the last 4 weeks of the study. Incidence and rates of hypoglycemia events throughout the study are shown in Table 4.
There was basically no difference in the incidence of hypoglycemia whether or not the patients had concomitant treatment with a GLP-1RA (48.4% with versus 45.9% without).
Other Evaluations
Regarding the DTSQ, treatment satisfaction increased by 20% between V1 and V3 with a 50% decrease in the perception of frequency of hyperglycemia and no change in the perception of frequency of hypoglycemia by the patients. Among the investigators, 85.3% and 87.9% agreed that they observed, at respectively V2 and V3, the anticipated impact of the treatment change according to the reason for insulin switch.
Discussion
The TRANSITION 2 study is the first real-world study in French patients with T2D switching to Gla-300. This interventional phase IV study could not be compared to the phase III EDITION 2 study [11] or the real-world evidence DELIVER 2 study [12] as these studies were different by design. EDITION 2 was a treat-to-target randomized open label study comparing Gla-100 and Gla-300 in patients with suboptimal glucose control on basal insulin and oral antidiabetic treatment. DELIVER 2 was a retrospective US cohort study looking at the effects of basal insulin switch (to Gla-300 or to another basal insulin) in T2D patients with suboptimal glucose control.
There were also differences regarding the population (participants were 64% men in TRANSITION 2, 46.3% in EDITION 2, 49% in DELIVER 2) and the baseline HbA1c (8.6 ± 0.9% in TRANSITION 2, 8.3 ± 0.9% in EDITION 2, 8.95% in DELIVER 2).
In TRANSITION 2, the inclusion criteria were relatively large compared to randomized trials and close to the real-life practice, as inclusion was limited to patients for whom a change in basal insulin was needed according to the investigator.
The reduction in HbA1c at 6 months was − 0.64% in TRANSITION 2 with 11.4% of the patients reaching an HbA1c value < 7.0% at 6 months. About 30% of patients reached an HbA1c value < 7.5%, a target accepted in clinical practice in France for insulin-treated patients with a long duration of diabetes and, for many of them, established cardiovascular disease. As it could be expected from studies with other antidiabetic agents [13], HbA1c reduction was greater in patients with higher baseline HbA1c level.
Even if the mean F-SMBG remained above the target range (90–130 mg/dL), 35% of the patients had reached this target at V2. It is possible that the real-life conditions led to a suboptimal titration in some patients, accounting for this relatively low percentage. The target range was set above the usual target that was used in the EDITION trials (80–100 mg/dL) as patients were already unsuccessfully treated with basal insulin with probable titration difficulties suggesting possible comorbidities and/or cognitive impairment. Also, in some of these patients, the progression of T2D may have required a treatment intensification to improve their glycemic control. However, the percentage of patients at target remained stable and slightly improved at V3, whereas the study duration was not long enough to assess if this would have had an impact on HbA1c later on.
Switching basal insulin in a patient with T2D is usually motivated by suboptimal glucose control and/or unacceptable frequency of hypoglycemia.
This study showed that difficulties in insulin titration represent another major difficulty for patients with T2D as it was one of the reasons for insulin switch in almost 40% of the patients. Titration is often a challenging issue and remains often inadequate in real life [14]. However, the adherence to the titration algorithm was quite good in this study as it was considered by the investigator as good or moderate in about 70.4% of the subjects at V2 and 74.2% at V3. This could explain why some patients were probably not encouraged to further titrate, as the investigators considered that they had reached the right target (38.4% at V3). Frequent contact with the investigator, including phone calls, has been shown to be very helpful [15] and a simple and easy titration algorithm probably contributed to the good adherence in this interventional trial. Also, the large increase in the daily insulin dose after V1 implies that titration was largely suboptimal with the previous insulin regimen. In the randomized trial EDITION 2, the dose of Gla-300 also increased until W12 and then stabilized.
Regarding safety issues, no adverse event was considered as being related to the basal insulin switch. The incidence of any hypoglycemia event during the study (46.0%) or nocturnal hypoglycemia (12.2%) can be compared to the respectively 43.6% and 11% prospective incidence of confirmed or severe hypoglycemia in a 30-day period in the French insulin-treated patients with T2D from the DIALOG real-life study [16] where various insulin regimens were used. It should also be noted that the incidence of nocturnal hypoglycemia (12.2%) was low compared to what can be observed in randomized trials such as EDITION 2 (30.5%). It cannot be excluded that the mandatory SMBG at 03:00 before each visit in the EDITION 2 randomized trial unmasked some hypoglycemia and motivated the patients to test more frequently at nighttime.
Furthermore, severe hypoglycemia was much less frequent in our study (2.2%) compared to the DIALOG study (6.4%), whereas HbA1c value was only slightly lower in the DIALOG study (7.9%) compared to TRANSITION 2 (8.1% at V2, 8.0% at V3). This is consistent with data from randomized trials showing less severe hypoglycemia with comparable HbA1c level in patients with T2D treated with Gla-300 even with impaired renal function [17], although this trend was not significant in all studies [18].
The rate of patient-reported hypoglycemia was the lowest before the switch of basal insulin when no insulin titration occurred. As expected, it increased afterwards. Surprisingly, the rate of hypoglycemia events appeared to increase during the last 4 weeks of the study, whereas the percentage of patients experiencing events was comparable to the titration period. This suggests that a higher number of events occurred in some subjects. Because of the low number of events and patients in the pre-defined HbA1c classes, we could not compare the ratio of hypoglycemia for each period of the study according to the HbA1c level. Nevertheless, global satisfaction was high among both patients and physicians. This was probably related to the improvement in HbA1c and the low rate of hypoglycemia with very few severe events. It is also possible that the insulin pen used with Gla-300 played a role in the improved satisfaction [19].
This study has some limitations. The main one is related to the design itself, with a single arm and thus no control group but it was a pragmatic study with very few exclusion criteria. The second could be the short duration of follow-up, but it was an interventional study with a quite large population. It is not relevant to compare the results of TRANSITION 2 study to the results of randomized trials [20]. Nevertheless, it is very important to collect real-life data as it is required by health authorities in France. Furthermore, local real-world studies provide evidence from clinically relevant real-life patient populations which may not be represented in RCTs with strict eligibility criteria.
The availability of antidiabetic drugs differs among countries and it is therefore important to have specific data [21]. In our study, almost half of the patients had concomitant treatment with a GLP-1RA. Although almost all of them had it before V1, it is probable that this treatment helped in achieving good glucose control in some patients [22] while there was no change in the rate of hypoglycemia and GLP-1RA treatment was not associated with a higher percentage of patients reaching a ≥ 0.3% HbA1c variation.
Conclusion
This 24-week interventional study is the first study conducted in close to real-life conditions with Gla-300 in France. A relatively large number of patients with uncontrolled T2D (HbA1c > 7.5% and F-SMBG > 130 mg/dL) on basal insulin with long-standing diabetes could be included and the adherence to the treatment and adherence to the algorithm titration were considered as good by the investigators. Results show a significant reduction in HbA1c (− 0.64%) after 6 months of treatment with about 30% of the patients reaching an HbA1c value < 7.5% and 50% reaching a value < 8.0%. HbA1c reduction was greater in patients with higher baseline value. Nearly 40% of patients had reached F-SMBG target range (90–130 mg/dL) as early as 12 weeks with reminders of the titration rules. Safety results in terms of adverse events and mostly hypoglycemia confirm previous data from randomized trials. Indeed, despite a clinically meaningful decrease of HbA1c, there were a limited number of hypoglycemic events and very few severe hypoglycemia events. Thus, Gla-300 appears, in close to real-life conditions, as an efficient and safe alternative in patients with uncontrolled T2D on basal insulin.
References
International Diabetes Federation. France: Country report 2017, The IDF Diabetes Atlas, 8th ed. http://reports.instantatlas.com/report/view/846e76122b5f476fa6ef09471965aedd/FRA. Accessed 26 Sept 2019.
Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7:e015949.
Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–76.
Riddle MC, Gerstein HC, Holman RR, et al. A1C targets should be personalized to maximize benefits while limiting risks. Diabetes Care. 2018;41:1121–4.
Ampudia-Blasco FJ, Benhamou PY, Charpentier G, et al. A decision support tool for appropriate glucose-lowering therapy in patients with type 2 diabetes. Diabetes Technol Ther. 2015;17:194–202.
Tiv M, Viel JF, Mauny F, et al. Medication adherence in type 2 diabetes: the ENTRED study 2007, a French population-based study. PLoS One. 2012;7:e32412.
Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28.
Balkau B, Calvi-Gries F, Freemantle N, Vincent M, Pilorget V, Home PD. Predictors of HbA1c over 4 years in people with type 2 diabetes starting insulin therapies: the CREDIT study. Diabetes Res Clin Pract. 2015;108:432–40.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:541–8.
American Diabetes Association. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55–64.
Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–43.
Zhou FL, Ye F, Berhanu P, et al. Real-world evidence concerning clinical and economic outcomes of switching to insulin glargine 300 units/mL vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20:1293–7.
Esposito K, Chiodini P, Maiorino MI, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open. 2015;5:e005892.
Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–96.
Bellido V, Bellido D, Tejera C, et al. Effect of telephone-delivered interventions on glycemic control in type 2 diabetes treated with glargine insulin. Telemed J E Health. 2019;25:471–6.
Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab. 2015;41:116–25.
Javier Escalada F, Halimi S, Senior PA, et al. Glycemic control and hypoglycemia benefits with insulin glargine 300 U/mL (Gla-300) extend to people with type 2 diabetes and mild-to-moderate renal impairment. Diabetes Obes Metab. 2018;20:2860–8.
Bolli GB, Riddle MC, Bergenstal RM, et al. Glycaemic control and hypoglycaemia with insulin glargine 300U/mL versus insulin glargine100U/mL in insulin-naïve people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43:351–8.
Klonoff D, Nayberg I, Erbstein F, Cali A, Brulle-Wohlhueter C, Haak T. Usability of the Gla-300 injection device compared with three other commercialized disposable insulin pens: results of an interview-based survey. J Diabetes Sci Technol. 2015;9:936–8.
Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–67.
Bloomgarden Z. Is insulin the preferred treatment for HbA1c > 9%? J Diabetes. 2017;9:814–6.
Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;20(384):2228–34.
Acknowledgements
The authors are very grateful to the patients for their committed participation in the study; they also thank all the investigators.
Funding
This study was funded by Sanofi. Sanofi also provided the funding for the Rapid Service Fee.
Editorial Assistance
Editorial support was provided by Sylvie Picard, MD, PhD (Endocrinology and Diabetes, Point Medical, Dijon, France). Funding for this assistance was provided by Sanofi France.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and their approval for this version to be published.
Disclosures
Pierre Gourdy has received occasional fees, either personally or institutionally, for the activities of speaker, scientific advisor or clinical research from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck Sharp & Dohme (MSD), Novartis, Novo Nordisk, Sanofi, Servier. Amar Bahloul is an employee of Sanofi. Zahra Boultif is an employee of Sanofi. Didier Gouet declares participation in punctual interventions (clinical trials, conferences or colloquiums, consulting) for companies Eli Lilly, Novo Nordisk, Sanofi Diabetes, Abbott, Medtronic, Janssen, Roche, MSD, AstraZeneca, Boehringer Ingelheim. Bruno Guerci declares activities of consultant, expert opinion, writing and proofreading; participation in phase II, III and IV clinical studies; speakers to symposia; co-funding or grants for clinical research projects for the following pharmaceutical and industrial firms, organizations and learned societies: Bristol-Myers Squibb, Sanofi Aventis, GlaxoSmithKline, Novartis, Novo Nordisk, Eli Lilly, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Janssen, Intarcia, Metacure, Pfizer, MSD, Roche Diagnosis, Medtronic, Menarini Diagnosis, Abbott, Lifescan, Vitalaire, Dinno Health, Ork’yn, ANSM, CNAMTS, CEPS, SFD, SFE and NSFA.
Compliance with Ethics Guidelines
All participants provided written informed consent and the study was conducted in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the revised Declaration of Helsinki, 1964 (ethical agreement number 1-15-22, CPP Sud-Ouest et Outre-Mer 1).
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10291166.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gourdy, P., Bahloul, A., Boultif, Z. et al. Efficacy and Safety of Switching Patients Inadequately Controlled on Basal Insulin to Insulin Glargine 300 U/mL: The TRANSITION 2 Study. Diabetes Ther 11, 147–159 (2020). https://doi.org/10.1007/s13300-019-00734-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00734-8