Abstract
Introduction
This research compares costs, resource utilization, and complications between adherent and nonadherent patients over the 3-year period post initiation on basal insulin therapy.
Methods
The study utilized the US-based Truven Health MarketScan® Research Databases from 2011 through 2015. Adults aged 18 years or older and identified with type 2 diabetes (T2D) who initiated therapy on basal insulin in 2012 were included. Patients were excluded if they were pregnant, filled their index basal insulin prescription via mail order, or were not continuously insured from 1 year before through 3 years following initiation of treatment with basal insulin. Instrumental variables were used to control for selection bias, and multivariable analyses were used to examine the associations between adherence to basal insulin therapy and costs, resource utilization, and acute complications.
Results
A total of 21,363 individuals were included in the study. Three years after initiating therapy on basal insulin, patients who were adherent over time to basal insulin treatment therapy (33.8% of patients) had significantly higher diabetes-related drug costs. However, patients’ adherence was associated with significantly lower diabetes-related outpatient, acute care, and total costs. Results for all-cause costs were similar. Adherent patients also had significantly fewer all-cause and diabetes-related hospitalizations and emergency room visits and were significantly less likely to be diagnosed with an acute complication.
Conclusions
Results of this study illustrate that despite higher drug costs, there are disease-specific and all-cause cost offsets and improved patient outcomes associated with adherence to basal insulin therapy for people with T2D.
Funding
Eli Lilly and Company.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 30 million American adults have diabetes mellitus (diabetes) [1], which in the USA is the leading cause of kidney failure, non-trauma related lower-limb amputations, and incident blindness [2]. Diabetes has also been found to be one of the major causes of heart disease, stroke, and death [2]. In 2012, the costs associated with diabetes totaled an estimated $245 billion in the USA, and such costs are forecasted to rise as the population ages [3]. Insulin is one of the suggested treatments for patients with type 2 diabetes (T2D) whose diabetes is not controlled with oral agents alone [4], although research has shown that over time most patients need multiple therapies to maintain glycemic control [5]. As it is long-acting, basal insulin is used to keep blood glucose level stable [6]. The American Diabetes Association states that basal insulin is the most convenient initial insulin regimen and is usually prescribed in combination with metformin and/or another noninsulin agent [4].
Previous research among patients with T2D has shown that basal insulin therapy is associated with a decrease in glycated hemoglobin (A1c) and fasting blood glucose as well as with improved treatment satisfaction [7], and treatment guidelines list basal insulin as the antidiabetic treatment with the highest efficacy [4]. However, physicians often delay initiation of insulin therapy among their patients with T2D [8]. Frequently cited reasons for this delay include patient concerns about the pain associated with blood testing and insulin injections, hypoglycemia, and weight gain associated with the use of insulin [9]. While patients have been reported to prefer simpler injection regimens [10], previous research has indicated that a large proportion of T2D patients discontinue their basal insulin therapy [11].
For patients with T2D, reduced adherence to drug therapy, in general, has been associated with poorer patient outcomes. Specifically, reduced adherence has been shown to be associated with increases in hospitalizations and emergency room (ER) visits [12,13,14,15], higher rates of complications [12, 16], and greater medical costs [17]. The goal of the present research was to compare costs, resource use, and complications between patients with T2D who were either adherent or nonadherent to basal insulin therapy within 3 years of initiating therapy. As such, the research will add to the body of evidence specifically related to the use of basal insulin and will examine adherence over an extended time horizon.
Methods
Data covering the period from 1 January 2011 through 21 December 2015 were obtained from the Truven Health MarketScan® Research Databases. Both the Commercial Claims and Encounters (CCAE) and Medicare Supplemental and Coordination of Benefits (MDCR) Databases were used in this study. The CCAE database consists of health insurance claims and patient enrollment data from large employers and health plans across the USA. The MDCR dataset represents health services received by employees, dependents, and retirees in the USA with primary or Medicare supplemental coverage. The CCAE and MDCR databases are generally representative of the population in the USA [18], and these databases provide detailed data on costs, medical resource use, and outcomes for healthcare services performed in both inpatient and outpatient settings. In addition to providing information about inpatient and outpatient services, medical claims are linked to outpatient prescription drug clams and person-level enrollment information. The data are fully de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). This article does not contain any new studies with human or animal subjects performed by any of the authors.
For entry into this study, patients were required to be identified as having T2D during the calendar year 2012. Such patients were identified if they received more diagnoses for T2D than for type 1 diabetes (T1D) and/or if they received a diagnosis for T2D and filled a prescription for an oral glucose-lowering agent (GLA) other than metformin or a sodium-glucose linked transport 2 inhibitor, since the latter agents may be used in the treatment of T1D [4, 19, 20]. Patients were furthermore required to have filled a prescription for basal insulin in 2012, with the date of the first fill identified as the index date. For each patient, data were included from 1 year before the index date (e.g., the pre-period) through 3 years after the index date (e.g., the post-period). Patients were excluded from the study if they: (1) filled a prescription for basal insulin during the pre-period, (2) were identified as pregnant at any time from the start of the pre-period through the end of the post-period, (3) were less than 18 years old as of the index date, (4) did not have continuous insurance coverage from the start of the pre-period through the end of the post-period, (5) did not have valid demographic data, or (6) received their index basal insulin prescription via mail order. The final sample consisted of 21,363 individuals. Figure 1 illustrates how each of the inclusion and exclusion criteria affected sample size.
The aim of the study was to examine the relationship between patient adherence to basal insulin as a class of therapeutics and outcomes, where adherence was proxied by the proportion of days covered (PDC). PDC was constructed as the percentage of days during the post-period that an individual had a supply of basal insulin, with adjustment for the possibility that insulin may not be used in a method consistent with the days’ supply field in a claims database [21]. A patient was considered to be adherent if the PDC reached the 80% threshold, and a patient was considered to be nonadherent if the PDC was < 80% [12, 15]. One advantage of using PDC as the measure of adherence is that it is used by both the Pharmacy Quality Alliance (PQA) and the Centers for Medicare and Medicaid Services (CMS) as a measure for examining the treatment of patients with diabetes [22, 23].
The patient outcomes of interest included medical costs, resource utilization, and acute complications. Costs were constructed using gross payments to a provider for a service, where payments were equal to the amount eligible for payment under the medical plan terms after applying rules such as discounts, but before applying coordination of benefits, copayments, and deductibles. All costs were converted to 2015 amounts using the medical component of the consumer price index. Both all-cause and diabetes-related costs were examined, and each of these cost categories was subcategorized to examine acute care (costs associated with hospitalizations or ER visits), outpatient costs, and prescription drug costs. All-cause costs consisted of all medical costs associated with inpatient, outpatient, and prescription drugs, and diabetes-related costs were constructed as the sum of all costs where (1) there was an accompanying primary or secondary diagnosis of diabetes; (2) there was receipt of a prescription for a GLA or diabetic supplies; or (3) the patient was identified as having hypoglycemia based upon a previously published and validated algorithm [24].
In addition to examining costs, the analyses also examined medical resource utilization and acute complications. The resource utilization outcomes examined included the probability of a hospitalization, the probability of an ER visit, the number of hospitalizations, the number of ER visits, and hospital length of stay (LOS). As with costs, resource utilization was categorized as either all-cause or diabetes-related. The study also examined the probability of being diagnosed with an acute complication over the 3-year post-period. An acute complication was identified based upon receipt of a primary or secondary diagnosis of hyperglycemia or diabetic coma or identification of hypoglycemia based upon the same algorithm used to identify the condition when constructing disease-specific costs [24].
When examining the relationship between adherence to basal insulin therapy and outcomes, the multivariable analyses controlled for factors that may potentially influence patient outcomes. Given the factors available in the database, the analyses controlled for patient demographic characteristics, general health and comorbidities, type of provider visited, medication use, and A1c tests. Patient demographic characteristics that were measured at the index date consisted of age, sex, region of residence, and insurance plan type. Patient overall general health was measured over the pre-period and assessed using the Charlson comorbidity index (CCI) [25], while, the Diabetes Complications Severity Index (DCSI) was utilized to proxy the severity of diabetes complications [26]. In addition to these index scores, the models also included pre-period comorbid diagnoses of anxiety, depression, and hyperlipidemia, since these diagnoses have been shown to be common in patients with diabetes or linked to patient nonadherence but were not captured in either of the two index scores [27, 28]. The analyses also controlled for visits to specialists in the pre-period with indicator variables for patient visits to a cardiologist, endocrinologist, ophthalmologist, or nephrologist, and they controlled for the type of basal insulin prescribed at the index date, the number of GLAs, and overall medication use. These factors were captured by an indicator variable if the index prescription was for U-500 basal insulin. As well, indicator variables captured the number of classes of GLAs prescribed in the pre-period and the number of non-GLA medications prescribed in the pre-period. Finally, the analyses controlled for the number of A1c tests the patient received in the pre-period. Consistent with previous research, this measure was used as a proxy for glycemic control [21], since laboratory results were unavailable in the database.
All multivariable models used the covariates discussed above, while the specific functional form depended upon the dependent variable being examined. In all cases, the estimated outcome was constructed from instrumental variables models, which are designed to allow for the role of unmeasured confounding. The use of retrospective data does not allow for patients to be randomized to treatment, potentially leading to sample selection bias. Instrumental variables are used to adjust for unmeasured confounding and the sample selection bias [29]. Consistent with previous research [30], the following variables were constructed over the first 30 days of the post-period and used as instruments: (1) the percentage of total payments for basal insulin prescriptions that were paid by coinsurance; and (2) the dollar (US) amount of copayments that were paid for basal insulin prescriptions. It is hypothesized that these variables will directly affect patient adherence while not being directly related to patient outcomes.
General linear models with gamma distribution and log link were used to estimate all costs models except those for acute care. Such general linear models have been shown to account for the skewed nature of cost data and to behave well in the estimation of population means of healthcare costs [31]. Acute care costs were estimated using a two-part model where, in the first part, a binary regression model was used to estimate the probability of having an acute care visit. In the second stage, a general linear model with gamma distribution and log link was used to estimate acute care costs for patients who had at least one acute care visit. The predicted probability of an acute care visit, estimated from the first part of the model, was then multiplied by costs estimates from the second part of the model to obtain unconditional average costs estimates.
Negative binomial models were used to examine the number of hospitalizations, the number of ER visits, and hospital LOS. Logistic models were used to examine the probability of being hospitalized or visiting the ER and the probability of being diagnosed with an acute complication.
Descriptive statistics were examined using Chi-square statistics for categorical variables and Kruskal–Wallis tests for continuous variables. For logistic models the odds ratios (ORs) and 95% confidence intervals (CIs) associated with being adherent (vs. nonadherent) were reported. For all other outcomes, the multivariable analyses were used to predict adjusted mean outcomes. Robust standard errors for each of these adjusted means were used to construct 95% confidence intervals for the adjusted means. Complete results of all multivariable analyses are presented in the supplementary material. All analyses were conducted using the SAS version 9.4 statistical software (SAS Institute, Cary, NC, USA). P values of < 0.05 were considered, a priori, to be statistically significant.
Results
Table 1 presents the descriptive statistics for the 21,363 T2D patients included in the study. The entire cohort’s mean age was 57 (standard deviation [SD] 12.67) years, and the majority were male (54.71%). Patients most commonly resided in the South (43.51%) or North Central (27.10%) regions of the USA, and most were covered by preferred provider organizations (56.15%) or had comprehensive insurance (15.02%). On average, patients filled prescriptions for 1.86 (SD 1.25) classes of GLAs in the pre-period and 9.32 (SD 6.30) prescriptions for non-GLA medications over the same period. Almost one-third of patients visited a cardiologist (30.85%), nearly one-quarter of patients visited an ophthalmologist (23.25%), and almost one-fifth visited an endocrinologist (17.27%) in the pre-period.
In addition to characterizing the entire population, Table 1 also presents descriptive statistics comparing adherent to nonadherent patients. Adherent patients were more likely to be male (57.36 vs 53.35%; P < 0.0001) and less likely to reside in the South (40.96 vs 44.81%; P < 0.0001), compared to nonadherent patients. Adherent patients were also in better general health than nonadherent patients, as evidenced by lower rates of anxiety (3.74 vs. 5.13%; P < 0.0001) and depression (6.96 vs. 8.98%; P < 0.0001), as well as by a significantly lower mean CCI score (2.27 [SD 1.84] vs 2.50 [SD 2.08]; P < 0.0001). A smaller percentage of adherent patients had visited a cardiologist (29.10 vs. 31.75%; P < 0.0001) in the pre-period compared to the nonadherent. In contrast, adherent patients were more likely to have been diagnosed with comorbid hyperlipidemia (57.07 vs. 51.82%; P < 0.0001) and to have visited an ophthalmologist (24.94 vs. 22.39%; P < 0.0001). Adherent patients may have had less severe diabetes compared to nonadherent patients, as evidenced by the significantly lower DCSI score (1.22 [SD 1.69] vs. 1.45 [SD 1.87]; P < 0.0001) and suggested by the larger number of A1c tests ordered for adherent patients (1.74 [SD 1.45] vs. 1.43 [SD 1.39]; P < 0.0001). The adherent patients were also prescribed more classes of GLAs in the pre-period (2.19 [SD 1.24] vs. 1.69 [SD 1.22]; P < 0.0001).
Figure 2 shows the relation between adherence to basal insulin and 3-year diabetes-related and all-cause medical costs. As shown in Fig. 2a, adherence to insulin therapy was associated with significantly lower diabetes-related outpatient costs ($6319 vs. $6759 [all costs shown in U.S. dollars]; P < 0.0001) and acute care costs ($8290 vs. $9789; P < 0.0001), but significantly higher diabetes-related drug costs ($11,898 vs. $10,316; P < 0.0001). Overall, diabetes-related total costs were significantly lower for adherent patients than for nonadherent patients ($26,488 vs. $26,800; P = 0.0227). All-cause costs generally follow the same pattern. Specifically, despite significantly higher drug costs over the post-period for the adherent patients relative to the nonadherent patients($22,267 vs. $21,030; P < 0.0001), the adherent cohort had significantly lower outpatient costs ($29,057 vs. $32,042; P < 0.0001), acute care costs ($22,112 vs. $25,458; P < 0.0001), and all-cause total costs ($73,687 vs. $78,778; P < 0.0001).
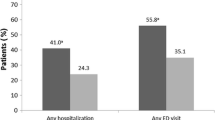
The associations between adherence and medical resource use are examined in Table 2 and Fig. 3. In general, these results indicate that adherence was associated with significantly better patient outcomes relative to nonadherence. As Fig. 3 illustrates, adherent patients were 29% less likely to have a diabetes-related hospitalization (OR 0.71; 95% CI 0.67–0.76) and 30% less likely to have a diabetes-related ER visit (OR 0.70; 95% CI 0.66–0.75). Similarly, adherent patients were 28% less likely to be hospitalized for any cause (OR 0.72; 95% CI 0.68–0.77), and 30% less likely to have an all-cause ER visit (OR 0.70; 95% CI 0.65–0.74). Consistent with the results shown in Fig. 3, Table 2 illustrates that the adherent patients had significantly fewer diabetes-related hospitalizations (0.53 [SD 0.45] vs. 0.64 [SD 0.58]; P < 0.0001) and ER visits (1.00 [SD 0.86] vs. 1.21 [SD 1.09]; P < 0.0001), as well as significantly fewer all-cause hospitalizations (0.73 [SD 0.69] vs. 0.89 [SD 0.85]; P < 0.0001) and all-cause ER visits (2.05 [SD 1.84] vs. 2.46 [SD 2.29]; P < 0.0001). Diabetes-related hospital LOS, all-cause hospital LOS, and the probability of an acute complication were also significantly lower for patients who were adherent to basal insulin therapy. Specifically, adherence to basal insulin therapy was associated with a 23% reduction in the probability of an acute complication being diagnosed over the post-period (OR 0.77; 95% CI 0.71–0.82).
Two sensitivity analyses were conducted to test the robustness of the results. First, all analyses were re-estimated using the medication possession ratio (MPR) rather than the PDC as the measure of adherence. In contrast to the PDC, the MPR is calculated using the sum of days’ supply for all prescriptions in a period of interest. As a result, this measure may overestimate adherence for patients who refill their prescriptions early. Second, PDC was recalculated without the adjustment to account for the fact that the days’ supply field filled out by the pharmacist may not be an accurate measure of how insulin is actually used by the patient. The findings were generally not sensitive to these alternative specifications, except that in both of the alternative models there were no statistically significant differences between the diabetes-related total costs of the adherent relative to the nonadherent patients.
Discussion
The findings support a large body of previous research that has revealed a link between GLA adherence in general and improved patient outcomes [12, 13, 15, 30, 32] as well as studies showing an association between persistence with basal insulin therapy and better outcomes [33, 34]. The present investigation furthers this research by focusing on basal insulin therapy over an extended time horizon while controlling for a wide range of factors that may affect patient outcomes. Additionally, this study examined the robustness of results to alternative measures of adherence.
Consistent with previous research [33, 34], adherence to basal insulin in the present study was associated with higher drug costs but lower acute care costs. Adherence was furthermore associated with lower total costs, a result that is in agreement with those of a number of studies that have looked at GLA adherence in general [17]. The present findings suggest that the increased medication costs associated with improved adherence were offset by decreases in acute care costs. It should be noted that diabetes-related total costs were not statistically significantly different between the two cohorts when alternative measures of adherence were examined.
In concert with earlier literature [12, 35,36,37], the adherent patients in the present study had a significantly lower use of hospital and ER resources relative to the nonadherent patients. This reduction in acute care costs represents potentially large costs savings for payers, given that hospitalization comprised 43% of all direct diabetes spending in the USA in 2012 [3]. Decreased use of acute care due to better adherence also has humanistic benefits, with less hospitalization associated with improved long-term outcomes, increased health-related quality of life [38], as well as reductions in indirect costs for patients and caregivers [3]. Hospitalization furthermore carries intrinsic health risks, including healthcare-associated infections [39, 40] and medical errors [41, 42]. Thus, spending on drug therapy that is associated with decreased use of acute care may be considered worthwhile in and of itself, let alone when offset by other cost reductions.
Relative to those who were nonadherent, the adherent cohort was significantly less likely to have an acute complication. This finding is consistent with previous research [12, 16] as well as with the finding in this study that adherence was associated with lower acute care costs. Research has also shown that prolonged exposure to hyperglycemia is associated with the development of diabetic complications [43], and among patients with one or more cardiovascular risk factor, severe hypoglycemia is associated with macrovascular events and cardiovascular mortality [44]. Furthermore, for older patients with T2D, research has found that hypoglycemic episodes accompanied with hospitalization or ER visits are associated with an increased risk of dementia [45]. A lower rate of acute complications over a 3-year time horizon may, therefore, have implications for longer term complications and outcomes.
The present study is similar in design to a previous one which examined adherence to GLAs in general over a 3-year time period [14]. The results are generally consistent between the two studies, although both all-cause and diabetes-related costs are higher in the present study and resource utilization in the post-period was higher. One key difference between the two studies is that this study included patients age 65 years or older, and these patients generally have more comorbidities and higher medical expenses. Furthermore, patients in the present study may have had T2D for a longer duration or more severe T2D, given that basal insulin therapy is not typically prescribed at the initiation of therapy but further along the path of disease progression.
The results of this study must be interpreted within the context of its limitations. First, the use of claims data does not allow for an examination of whether the medication is being taken as prescribed, or even taken at all. This limitation may be especially important when examining the use of insulin, which is more challenging to administer than oral drugs. Also, all patients who stopped taking insulin in the post-period were identified as having reduced adherence, since the reason for discontinuation of therapy is not known. As a result, patients who switched or discontinued therapy due to adverse events were categorized as nonadherent. However, it should be noted that this measurement and terminology are consistent with a wide range of previous research which has examined the association between the use of insulin and patient outcomes [46,47,48,49].
In addition, the analyses were based upon observational health insurance claims data that described a population of commercially insured T2D patients who may or may not be representative of the majority of Americans with T2D. For example, the population does not include patients who were uninsured or insured via Medicaid or through the Veterans Health Administration. Research has shown that both insurance type and the lack of insurance affect patient adherence [50, 51]. The data also overrepresent patients from the Southern and North Central regions of the USA [52], although the multivariable analyses controlled for regional differences. The use of diagnostic codes was not as rigorous as formal assessments and may underrepresent certain conditions, such as hypoglycemia. Factors such as A1c, race, duration of diabetes, or socioeconomic class, may be associated with patient outcomes but could not be controlled for as they were not observable in our database. However, the instrumental variables models were used to control for unobservable confounders. Finally, the study focused on statistical significance and was unable to determine whether differences in outcomes represented minimal clinically important changes.
Conclusions
In conclusion, these analyses showed that patients who adhered to their basal insulin therapy over a 3-year time horizon had significantly lower total costs (diabetes-related and all-cause) relative to their nonadherent counterparts, despite having higher drug costs. In this study, basal insulin adherence was furthermore associated with lower diabetes-related and all-cause outpatient costs and acute care costs, lower odds of an acute complication, lower odds or a hospitalization or ER visits, fewer hospitalizations and ER visits, and shorter hospital LOS. The findings of this study suggest that for adults with T2D adherence to basal insulin may lead to significant patient benefits while reducing costs for payers.
References
American Diabetes Association. Statistics about diabetes [Internet]. 2017. http://www.diabetes.org/diabetes-basics/statistics/. Accessed December 2017.
Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011 [Internet]. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed December 2017.
American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033–46.
American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41[Suppl 1]:S73–85.
Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–12.
Kuroda A, Kaneto H, Yasuda T, et al. Basal insulin requirement is 30% o. f the total daily insulin dose in type 1 diabetic patients who use the insulin pump. Diabetes Care. 2011;34(5):1089–90.
Chien M, Chen Y, Hung Y, et al. Glycemic control and adherence to basal insulin therapy in Taiwanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2016;7(6):881–8.
Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care. 2014;37(6):1499–508.
Lau ANC, Tang T, Halapy H, Thorpe K, Yu CHY. Initiating insulin in patients with type 2 diabetes. CMAJ. 2012;184(7):767–76.
Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–5.
Westerbacka J, Mihailov H, Valle T, Jääskeläinen S, Kaukua J. Adherence to initiated basal insulin analog treatment in type 1 and 2 diabetes. Value Health. 2015;18(7):A612.
Gibson TB, Song X, Alemayehu B, et al. Cost sharing, adherence, and health outcomes in patients with diabetes. Am J Manag Care. 2010;16(8):589–600.
Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). 2012;31(8):1836–46.
Curtis SE, Boye KS, Lage MJ, Garcia-Perez L-E. Medication adherence and improved outcomes among patients with type 2 diabetes. Am J Manag Care. 2017;23(7):e208–14.
Juarez DT, Tan C, Davis J, Mau M. Factors affecting sustained medication adherence and its impact on health care utilization in patients with diabetes. J Pharm Health Serv Res. 2013;4(2):89–94.
Yu AP, Yu YF, Nichol MB. Estimating the effect of medication adherence on health outcomes among patients with type 2 diabetes—an application of marginal structural models. Value Health. 2010;13(8):1038–45.
Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–17.
Hansen L. The Truven Health MarketScan Databases for life sciences researchers[Internet]. 2017. https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf. Accessed March 2018
Livingstone R, Boyle JG, Petrie JR. REMOVAL study team. A new perspective on metformin therapy in type 1 diabetes. Diabetologia. 2017;60(9):1594–600.
El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;137:83–92.
Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27(9):1709–17.
Pharmacy Quality Alliance. PQA medication quality measures in the health insurance marketplace [Internet]. 2017. http://pqaalliance.org/measures/qrs.asp. Accessed December 2017.
Academy of Managed Care Pharmacy. Medicare part D quality measures [Internet]. 2014. http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=18900. Accessed December 2017
Ginde A, Blanc P, Lieberman R, Camargo C. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8(1):4.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Chang H-Y, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721–6.
Ayyagari R, Wei W, Cheng D, Pan C, Signorovitch J, Wu EQ. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health. 2015;18(2):198–205.
Palizgir M, Bakhtiari M, Esteghamati A. Association of depression and anxiety with diabetes mellitus type 2 concerning some sociological factors. Iran Red Crescent Med J. 2013;15(8):644–8.
Basu A. Estimating decision-relevant comparative effects using instrumental variables. Stat Biosci. 2011;3(1):6–27.
Encinosa WE, Bernard D, Dor A. Does prescription drug adherence reduce hospitalizations and costs? The case of diabetes. Adv Health Econ Health Serv Res. 2010;22:151–73.
Malehi AS, Pourmotahari F, Angali KA. Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Econ Rev. 2015;5:11.
Cheng S-H, Chen C-C, Tseng C-H. Does medication adherence lead to lower healthcare expenses for patients with diabetes? Am J Manag Care. 2013;19(8):662–70.
Hadjiyianni I, Desai U, Suzuki S, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation: a retrospective database study among people with Type 2 diabetes mellitus in Japan. Diabetes Ther. 2017;8(1):149–66.
Perez-Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32(4):669–80.
Wong ES, Bryson CL, Hebert PL, Liu C-F. Estimating the impact of oral diabetes medication adherence on medical costs in VA. Ann Pharmacother. 2014;48(8):978–85.
Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109.
Han E, Suh D-C, Lee S-M, Jang S. The impact of medication adherence on health outcomes for chronic metabolic diseases: a retrospective cohort study. Res Soc Adm Pharm. 2014;10(6):e87–98.
Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc. 2009;57(3):395–402.
Sydnor ERM, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24(1):141–73.
Quach C, McArthur M, McGeer A, et al. Risk of infection following a visit to the emergency department: a cohort study. CMAJ. 2012;184(4):E232–9.
Gleason KM, McDaniel MR, Feinglass J, et al. Results of the medications at transitions and clinical handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–7.
Bleetman A, Sanusi S, Dale T, Brace S. Human factors and error prevention in emergency medicine. Emerg Med J. 2012;29(5):389–93.
Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16.
Haukka J, Hoti F, Erästö P, Saukkonen T, Mäkimattila S, Korhonen P. Evaluation of the incidence and risk of hypoglycemic coma associated with selection of basal insulin in the treatment of diabetes: a Finnish register linkage study. Pharmacoepidemiol Drug Saf. 2013;22(12):1326–35.
Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565–72.
Chandran A, Bonafede MK, Nigam S, Saltiel-Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148–58.
Cobden D, Lee WC, Balu A, Joshi V, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948–62.
Kleinman NL, Schaneman JL, Lynch WD. The association of insulin medication possession ratio, use of insulin glargine, and health benefit costs in employees and spouses with type 2 diabetes. J Occup Environ Med. 2008;50(12):1386–93.
Egede LE, Gebreqziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533–9.
Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7(35–44):3.
Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche-Orlow MK, Schnipper JL. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. 2012;7(6):470–5.
US Census Bureau. United States population growth by region [Internet]. 2017. https://www.census.gov/popclock/data_tables.php?component=growth. Accessed March 2018.
Acknowledgements
Funding
Sponsorship for this study and article processing charges was funded by Eli Lilly and Company.
Editorial Assistance
The authors would like to thank Patricia Platt and Michael Treglia of HealthMetrics Outcomes Research for their assistance in manuscript preparation.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Magaly Perez-Nieves is an employee and shareholder of Eli Lilly and Company. Kristina S. Boye is an employee and shareholder of Eli Lilly and Company. Jacek Kiljanki is an employee and shareholder of Eli Lilly and Company. Dachung Cao is an employee and shareholder of Eli Lilly and Company. Maureen J Lage is an employee of HealthMetrics Outcomes Research, which received consulting fees from Eli Lilly and Company for her work on this project.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. All patients were de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act.
Data Availability
The datasets analyzed during the current study are not publicly available due to licensing agreements between Truven and Eli Lilly and Company but are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.6026558.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Perez-Nieves, M., Boye, K.S., Kiljanski, J. et al. Adherence to Basal Insulin Therapy Among People with Type 2 Diabetes: A Retrospective Cohort Study of Costs and Patient Outcomes. Diabetes Ther 9, 1099–1111 (2018). https://doi.org/10.1007/s13300-018-0421-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0421-5