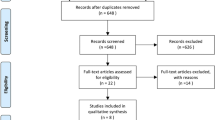
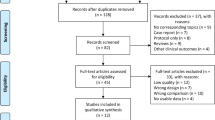
Abstract
Osteosarcoma is the most common malignant bone tumor, and the prognosis of patients with osteosarcoma is still unsatisfactory with low survival rates. There are many studies assessing the prognostic role of upregulated p53 in patients presenting osteosarcoma, and there is no consistent finding. To summarize the existing evidence about whether the presence of upregulated p53 was a biomarker of survival in patients with osteosarcoma, we performed a systematic review and meta-analysis of relevant publications. We assessed the effect of upregulated p53 on the 3-year overall survival and the 3-year disease-free survival by calculating the pooled odds ratio (OR) with corresponding 95 % confidence interval (95 %CI). Fifteen studies with a total of 609 patients with osteosarcoma were finally included into the systematic review and meta-analysis. Compared with osteosarcoma patients with low or undetectable p53, patients with upregulated p53 were obviously associated with decreased 3-year overall survival (OR = 0.29, 95 %CI 0.19–0.43, P < 0.001). In addition, patients with upregulated p53 were obviously associated with decreased 3-year disease-free survival (OR = 0.06, 95 %CI 0.02–0.23, P < 0.001). The results from the systematic review and meta-analysis highlight that p53 is an effective biomarker of survival in patients with osteosarcoma. In addition, more studies with a large sample size are needed to identify the effect of p53 expression in osteosarcoma patients.



Similar content being viewed by others
References
Bielack S, Carrle D, Casali PG. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:137–9.
Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92.
Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–9.
Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–63.
Janeway KA, Grier HE. Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol. 2010;11:670–8.
Ye ZM, Li WX, Fan SW, Yang DS, Tao HM. Expression of p53 protein and proliferating cell nuclear antigen (PCNA) in osteosarcoma and its prognostic significance [article in Chinese]. Zhejiang Med J. 2000;22:65–7.
Zhang L, Yang ZY, Tang RY, Mei J, Ai ZS. The relationship with the protein expression of p53, pten and the three-year survival ratios in osteosarcoma patients [article in Chinese]. J Pract Radiol. 2003;19:673–6.
Wu X, Chen ZR, Zhang GJ. [Apoptosis-related gene expression and its clinical significance of human osteosarcoma]. Zhonghua Zhong Liu Za Zhi. 2004;26:678–81.
Bao Y, Li HP, Fan R, Jiang C. Expression of p53 protein in osteosarcoma and its significance [article in Chinese]. Acta Acad Med CPAPF. 2006;15:230–1.
Hu X, Yu AX, Qi BW, Fu T, Wu G, Zhou M, et al. The expression and significance of idh1 and p53 in osteosarcoma. J Exp Clin Cancer Res. 2010;29:43.
Zhang SQ, Xiao DM, Zhu YH, Guan H, Li W, Tan JF. Protein expression of p53 in osteosarcoma and the correlation with clinical features and prognosis [article in Chinese]. J Mod Oncol. 2011;19:145–6.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Boulytcheva IV, Soloviev YN, Kushlinskii NE, Mahson AN. Expression of molecular markers in the tumor and survival prognosis in osteosarcoma. Bull Exp Biol Med. 2010;150:237–42.
Ferrari S, Bertoni F, Zanella L, Setola E, Bacchini P, Alberghini M, et al. Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004;100:1936–42.
Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–8.
Kaseta MK, Khaldi L, Gomatos IP, Tzagarakis GP, Alevizos L, Leandros E, et al. Prognostic value of bax, bcl-2, and p53 staining in primary osteosarcoma. J Surg Oncol. 2008;97:259–66.
Oliveira CR, Mendonca BB, Camargo OP, Pinto EM, Nascimento SA, Latorre Mdo R, et al. Classical osteoblastoma, atypical osteoblastoma, and osteosarcoma: a comparative study based on clinical, histological, and biological parameters. Clinics (Sao Paulo). 2007;62:167–74.
Ozger H, Eralp L, Atalar AC, Toker B, Esberk Ates L, Sungur M, et al. [The effect of resistance-related proteins on the prognosis and survival of patients with osteosarcoma: an immunohistochemical analysis]. Acta Orthop Traumatol Turc. 2009;43:28–34.
Papai Z, Feja CN, Hanna EN, Sztan M, Olah E, Szendroi M. P53 overexpression as an indicator of overall survival and response to treatment in osteosarcomas. Pathol Oncol Res. 1997;3:15–9.
Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–73.
Wu X, Cai ZD, Lou LM, Zhu YB. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol. 2012;36:212–6.
Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–23.
Vousden KH, Ryan KM. P53 and metabolism. Nat Rev Cancer. 2009;9:691–700.
Silva JL, Vieira TC, Gomes MP, Bom AP, Lima LM, Freitas MS, et al. Ligand binding and hydration in protein misfolding: insights from studies of prion and p53 tumor suppressor proteins. Acc Chem Res. 2010;43:271–9.
Fan C, Lin X, Wang E. Clinicopathological significance of cathepsin d expression in non-small cell lung cancer is conditional on apoptosis-associated protein phenotype: an immunohistochemistry study. Tumour Biol. 2012;33:1045–52.
Liu J, Ma Q, Zhang M, Wang X, Zhang D, Li W, et al. Alterations of tp53 are associated with a poor outcome for patients with hepatocellular carcinoma: evidence from a systematic review and meta-analysis. Eur J Cancer. 2012;48:2328–38.
Scoccianti C, Vesin A, Martel G, Olivier M, Brambilla E, Timsit JF, et al. Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: the EUELC cohort. Eur Respir J. 2012;40:177–84.
Acknowledgments
This research was supported by grants from the Heilongjiang Provincial Education Department (no. 12511205) and Heilongjiang Provincial Health Department (no. 2011–062).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, HL., Shao, L., Wang, Q. et al. A systematic review of p53 as a biomarker of survival in patients with osteosarcoma. Tumor Biol. 34, 3817–3821 (2013). https://doi.org/10.1007/s13277-013-0966-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0966-x