Abstract
Background
Norcantharidin (NCTD) has multiple antitumor effects. However, NCTD can induce significant hepatotoxicity and the mechanism of hepatotoxicity is not clear for now.
Objective
This study aimed to explore the hepatotoxicity of NCTD in rat by ultra-performance liquid chromatography (UPLC) quadrupole time-of-flight (Q-TOF)-MS (UPLC/Q-TOF-MS) metabolomics.
Results
Serum biochemical indices including alanine aminotransferase (ALT) and total bilirubin (T-BIL) were significantly increased. Histopathological and ultrastructure results revealed that hepatocytes were damaged. Furthermore, the metabolomics results showed that 11 metabolites in serum and 8 metabolites in liver were differential metabolites for NCTD hepatotoxicity. Four metabolic pathways including the sphingolipid metabolism, purine metabolism, arachidonic acid metabolism, and glycerophospholipid metabolism were the key metabolic pathways related to NCTD hepatotoxicity.
Conclusion
The metabolomics analysis in this study reveal new clues on the hepatotoxicity mechanism of NCTD in rats. These findings have potential applications in the toxicity study of NCTD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver is a frequent target for drug toxicity because it is the site of xenobiotic metabolism, and xenobiotic compounds may be directly or indirectly toxic to the hepatocyte in their native form or after metabolism by the liver (Losser and Payen 1996). Drug induced liver injury refers to the abnormal liver function caused by the drug itself and/or its metabolites during treatment. Recent studies have found that direct hepatotoxicity, oxidative stress, mitochondrial dysfunction and immune response might be the potential mechanisms of most drug-induced liver injury (Katarey and Verma 2016; McGill and Jaeschke 2019).
Norcantharidin (NCTD), a 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid derivative, is the demethylated synthetic analog of cantharidin. NCTD has significant antitumor effect primarily by interrupting the cell cycle progression, inhibiting tumor metastasis and inducing cellular apoptosis (Chen et al. 2018; Pachuta-Stec et al. 2019; Zhou et al. 2020). NCTD is commonly used as combined chemotherapy drug for treating primary hepatocellular carcinoma, esophageal cancer and leucopenia (Jiang et al. 2017; Wang et al. 2018; Pan et al. 2020; Singh et al. 2021).
Many studies have shown that NCTD has short biological half-life and rapid elimination in vivo. Therefore, high-dose administration or alternative dosage forms such as NCTD microspheres, liposomes and nanoparticles were usually used in clinical, which could improve the effectiveness and safety of NCTD treatment. However, the application of high dose of NCTD or the use of new dosage forms of NCTD produced severe liver toxic effects (Liu et al. 2017; Ma et al. 2018). For example, a previous study have pointed out that rats showed hepatic failure after intragastric administration of NCTD at the dose of 15.0 mg/kg (Lin et al. 2012). Moreover, studies have demonstrated that NCTD can be absorbed rapidly into various organs, and the distribution of NCTD in liver was relatively high and showed a dose-dependent manner, which suggested the accumulation of NCTD in liver leads to hepatotoxicity (Ding et al. 2012; Chi et al. 2019). Further study has demonstrated that the liver developed necrosis and inflammation on exposure to NCTD, and the endoplasmic reticulum and mitochondria were damaged after the administration of NCTD in mice (Gao et al. 2021). However, like other drugs with hepatotoxicity, NCTD hepatotoxicity was latent and difficult to be found, and the specific toxic mechanism of NCTD was not clear.
Metabolomics, as an important branch of emerging omics technology, has been widely used in the toxicity research of traditional Chinese medicine (Zhang et al. 2012; Tawfike et al. 2013). Metabolomics can sensitively identify a number of low-molecular-weight endogenous metabolites in the biological samples in response to different stimuli, so as to providing novel and specific biomarkers for drug-induced injury and for predicting toxicity (Rawat et al. 2016). Liquid chromatography-mass spectrometry (LC/MS) is suitable for nontargeted metabolomics study because of its wide dynamic range and chemical diversity coverage (Yan et al. 2020). Hence, in this study, the hepatotoxicity of NCTD in rats was studied by combining traditional hepatotoxicity evaluation index and LC-MS metabolomics technology, which will provide effective information for future research of NCTD.
Materials and methods
Chemicals and reagents
NCTD (purity > 98.0%, lot: D1601204) was purchased from Aladdin Industrial Corporation (Shanghai, China). Sodium Carboxymethyl cellulose (lot: 712O022) and 10% neutral-buffered formalin (lot: 20,180,419) was bought from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Methanol (LC/MS grade, lot: 172,471) and water (LC/MS grade, lot: 160,612) were purchased from Fisher Scientific (Fisher Scientific, Fair Lawn, NJ, USA), acetonitrile (LC/MS grade, lot: 172,471) was purchased from Merck (Merck, Darmstadt, Germany), formic acid (LC/MS grade, lot: 56,302) was purchased from Sigma-Aldrich (Sigma-Aldrich Japan, Chiba, Japan).
Animals
Twenty-four Sprague–Dawley rats (half male and female) weighing 180 ± 20 g were purchased from Changsha Topgene Bio-technology Co., Ltd. (Changsha, Hunan, China; SCXK (HN) 2014–0011). All rats were housed in a 12 h light–dark room for one week of acclimatization. The temperature and humidity were regulated at 25 ℃ and 40%, respectively. All protocols were approved by the Institutional Committee on Animal Care and Use of Zunyi Medical University (ZMUER2017-2-235).
Animal experiment
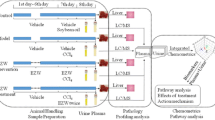
All rats were randomly divided into three groups (n = 8, half male and female) as follows: control group, NCTD low-dose group (NCTDL, 0.3 mg/kg/day) and NCTD high-dose group (NCTDH, 1.5 mg/kg/day). The selection of NCTD doses was based on previous study and was in conformity with the principle of experimental zoology (Xu et al. 2003; Fan et al. 2017). Different concentrations of NCTD were obtained by dissolving NCTD in 0.5% sodium carboxymethyl cellulose solution. The rats in NCTD group were administered with NCTD solution by gavage, and those in the control group were administered with 0.5% sodium carboxymethyl cellulose solution (1.0 mg/kg/day). The administration lasted for 15 days and all the rats were weighed after the experiment.
Biological sample collection
2 mL blood of rats was drawn from the orbital sinus using micro-capillary tubes at 1, 5, 10, and 15 days after intragastric administration. All the blood samples were centrifuged for 10 min (11,000 g, 4 ℃), then serum was obtained for biochemical analysis. Furthermore, serum at 15 days was stored at − 80 ℃ for metabolomic analysis. After the last time of blood collection, all rats were anesthetized by 20% ethyl carbamate (1.0 g/kg, intraperitoneally) and dissected, and then liver tissues of all rats were immediately removed out and rinsed with normal saline. The liver tissues of 6 rats (half male and female) from each group were used for histopathological examination and metabolomic analyses, liver tissues of 2 rats (half male and female) from each group were used for ultrastructure analysis.
Liver coefficient and biochemical assays
All rats were administered of NCTD continuously for 15 days. After the last administration, the body weights of rats were weighed, and the weights of liver tissues were also recorded after dissection. The liver-to-body weight ratio (liver coefficient) was calculated as follows:
Liver biochemical indices including ALT, T-BIL and LDH in the serum were measured using an automatic blood biochemical analyzer (Beckman Coulter, Inc., Brea, CA, USA).
Histopathological examination and ultrastructure analysis
6 liver tissue was rinsed with precooled normal saline, dried using filter paper and weighed. An aliquot of the liver tissues was fixed in 10% neutral-buffered formalin for 24 h, embedded in paraffin wax, sliced into serial sections of 5 µm thicknesses, then stained with hematoxylin and eosin, and observed under light microscopy for histopathological examination. 2 liver tissues was fixed with 3% glutaraldehyde solution for 30 min and cut into pieces measuring 1 × 1 × 2 mm. Finally, the ultrastructure of the hepatocytes was observed by transmission electron microscopy after staining with uranium acetate and lead citrate.
Metabolomic analysis
Sample preparation
The serum samples were thawed at 4 ℃, after which 300 µL of methanol was added to each 100 µL of serum for protein precipitation. Then, the mixture was used for vortex (30 s) and centrifuged at 11,000 g and a temperature of 4 ℃ for 10 min. Next, the supernatant was removed and analysed by UPLC/Q-TOF-MS.
Liver samples weighing 150 mg were added with 1 mL homogenization buffer (methanol: acetonitrile: water at 2:2:1 v/v/v) using the SCIENTZ-48 tissue grinder (SCIENTZ, Zhejiang, China). After centrifugation at 11,000 g, 4 ℃ for 10 min, the supernatant was transferred and kept overnight at − 20 ℃. The supernatant was centrifuged again for 10 min (11,000 g, 4 ℃). Then, 600 μL supernatant was transferred into UPLC/Q-TOF-MS for analysis. Quality control (QC) samples were prepared in parallel by mixing 20 μL of each sample supernatant to check the stability of the UPLC/Q-TOF-MS system.
Chromatographic and mass spectrometric conditions
The metabolites in the serum and liver were analysed using the previously developed UPLC/Q-TOF-MS system (Zhang et al. 2020). The Triple TOF™ 4600 system (SCIEX, Framingham, MA, USA) fitted with an LC 30 AD system (Shimadzu, Kyoto, Japan) was used. An Agilent ZORBAX Eclipse XDB-C18 column (100 × 2.1 mm, 1.8 μm) was used in the positive mode with solvent A (0.1% formic acid/water, v/v), and solvent B (acetonitrile); the gradient program was: 0–1.0 min, 5% B; 1.0–6.0 min, 5–65% B; 6.0–18.0 min, 65–100% B; 18.0–20.1 min, 100–5% B; and 20.1–25.0 min, 5% B. The Waters ACQUITY UPLC® HSS T3 column (100 × 2.1 mm, 1.8 μm) was used in the negative mode with solvent A (water), and solvent B (acetonitrile). A flow rate of 0.3 mL/min was used, and the injection volume was 3 μL for both modes. MS was conducted from 100 to 1000 m/z. The capillary temperature was 600 ℃. The ion spray voltage was 5500 V in the positive mode and − 5500 V in the negative mode.
Data processing and analysis
The raw data obtained from UPLC/Q-TOF-MS were exported into OSI/SMMS software 2.0 (Dalian ChemDataSolution Information Technology Co. Ltd, Dalian, China) to identify metabolites. The half-peak width was set to 5, meanwhile, format conversion, peak recognition, peak alignment, matching, and identification were processed using MS1 and MS2 intensity. Finally, an excel table consist of mass charge ratio, retention time, corresponding intensities and identification results for all detected peaks was obtained.
Next, the data set after the above processing was imported into SIMCA 14.1 (Umetrics, Umeå, Sweden) for multivariate data analysis. Principal component analysis (PCA) was used to identify clustering patterns and screen outliers. Partial least-squares discriminant analysis (PLS-DA) was conducted to visualize discrimination between the NCTD and control groups and to find alterations in metabolic profiling induced by NCTD. The predictability and effectiveness of the model was assessed by R2X, R2Y and Q2Y. Response permutation test were performed 200 times to avoid over fitting in the supervised orthogonal partial least-squares discriminant analysis (OPLS-DA) model. Variable importance in projection (VIP) values produced in the OPLS-DA model was then used to identify differential metabolites. Metabolites with VIP > 1 were further processed using Student’s test and fold-change (FC) values. Metabolites with VIP > 1, P < 0.05, and FC > 2 or < 0.5 were considered as differential metabolites.
Finally, the KEGG database (https://www.kegg.jp/) and MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/MetaboAnalyst/ModuleView.xhtml) were applied to obtain the KEGG ID of metabolites and analyse important metabolic pathways.
Statistical analysis
The data were presented as the mean ± SD and analyzed using the SPSS software (v.18.0). The significance of the differences was determined by one-way analysis of variance and Fisher’s least significant difference tests. The Student’s t test was used to evaluate the significant differences in differential metabolites. P < 0.05 was considered statistically significant.
Result
Liver coefficient and biochemical analysis
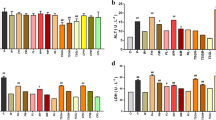
NCTD-induced liver injury in rats was assessed by evaluating liver coefficient and biochemical indices. The liver coefficient of rats in the NCTD groups was increased compared with the control group (Fig. 1A). The result of liver coefficient suggested that NCTD caused liver injury in the rats.
After 15 days administration of NCTD, the ALT level increased at 5, 10, 15 days, and it was significantly increased in NCTDH group compared with the control group (Fig. 1B). The TBIL level significantly increased at 1, 5, 15 days in NCTDH group compared with control group (Fig. 1C). All these increased biochemical indices suggested that the liver was damaged by high dose of NCTD. The level of LDH in NCTD group was increased at 1 day and decreased at 15 day compared with control group (Fig. 1D).
Histopathological and ultrastructure analysis
After 15 days administration, histopathological results showed that hepatocytes in the control group were arranged radially with the central vein as the center, and the structures of hepatic cord and sinusoid were obvious (Fig. 2A). The NCTDL group showed that the hepatic sinusoids were slightly dilated and the hepatic cords were disordered (Fig. 2B). In NCTDH group, the hepatic sinusoids were dilated, and the hepatocytes around the central vein were slightly swollen. Besides, obvious lipid vacuolation were observed in NCTDH group, and the hepatocytes around the center of the lobule appeared slightly edema and hypertrophy (Fig. 2C).
As shown in Fig. 3A, in control group, the structure of the hepatocytes was intact, the nuclei of the hepatocytes were normal in shape and the endoplasmic reticulum was neatly arranged. In NCTDL group, the endoplasmic reticulum was slightly dilated the cytoplasm and showed rarefaction and vacuolation (Fig. 3B). In the NCTDH group, the endoplasmic reticulum was severely dilated and the perinuclear space was wider. Moreover, most of the cell organelles degenerated and a large number of lipid vacuoles were observed (Fig. 3C). Based on the above results, the hepatocytes were damaged after administration of NCTD.
QC sample detection and system stability analysis
The repeatability and stability of the UPLC/Q-TOF-MS system was evaluated by analysing QC samples. Representative total ion current chromatograms (TIC) of serum and liver tissue QC samples in both negative and positive ion modes are shown in Figs. 4 and 5, respectively. TIC results showed that the retention time (RT) and peak intensity of the metabolites detected in QC samples showed considerable overlapping. These results indicate that the detection system was stable and repeatable.
Furthermore, to assess the instrument precision and system stability, three fragment ions with a mass-to-charge ratio (m/z) between 100 and 1000 and an RT cover in cycle were selected based on a previous method (Zhang et al. 2020). Analysing each data spiked with metabolite in different QCs revealed different levels of information about the QCs (Godzien et al. 2014). Results showed that the relative standard deviation (RSD) of serum QC samples under positive and negative ions was < 12.08 and 7.88%, respectively. The RSD of liver QC samples under positive and negative ions was < 8.00 and 10.60%, respectively, which indicated that the instrument was stable and the error was minimal during the entire sample data acquisition experiment (Table 1).
Multivariate statistical analysis
A total of 3968 variables in the serum were detected under the positive and negative modes. In liver samples, 3541 variables were detected under the positive and negative modes. All the variables were used for the multivariate analysis.
First, PCA was used to observe inherent clustering to identify possible outliers. In rat serum, good separation was observed between control and NCTD groups in PCA score plot, and the cumulative interpretation rate of the model in the x-axis direction, that is R2X(cum) was 0.582 (Fig. 6A). The variance explained by the first two components R2X[comp1] and R2X[comp2] were 0.2233 and 0.332, respectively (Fig. 6B). QC samples clustered tightly, illustrating the stability of the UPLC-QTOF/MS platform throughout the analysis.
Shown as Fig. 6C and D, PLS-DA was used to identify the disturbed metabolites in biological system. The quality and reliability of the PLS-DA models were validated based on R2X, R2Y and Q2 values derived using sevenfold cross validation algorithm. R2X, R2Y and Q2 were 0.457, 0.938 and 0.784, respectively. The F value of the cross-validated ANOVA and corresponding P value were 8.80244 and 0.000013, respectively. The result indicated that the PLS-DA model possessed satisfactory fit with good prediction. The differential metabolites were selected based on VIP values (VIP > 1).
For a deeper explanation of the differential variables between the NCTDH and control groups, OPLS-DA analysis was conducted. The score plots of OPLS-DA showed an obvious separation between the NCTDH and control groups (Fig. 6E), R2X, R2Y and Q2 were 0.364, 0.998 and 0.951, respectively. Furthermore, a 200 permutation test was used to validate the OPLS-DA models. Result of the R2 and Q2 values on the left was lower than the original R2Y and Q2 points on the right, which indicated that the OPLS-DA model in rat serum were efficient (Fig. 6F).
Similarly, in rat liver, the PCA score plot also showed good separation between control and NCTD groups. The R2X(cum), R2X[comp1] and R2X[comp2] were 0.534, 0.226 and 0.351, respectively (Fig. 7A and B). The R2X, R2Y and Q2 in PLS-DA model were 0.464, 0.945 and 0.817, respectively. The F value of the cross-validated ANOVA and corresponding P value were 8.16532 and 0.000038, respectively (Fig. 7C and D). The R2X, R2Y and Q2 in OPLS-DA model were 0.329, 0.986 and 0.863, respectively. Result of the 200 permutation test also showed that the OPLS-DA model was efficient in rat liver (Fig. 7E and F).
The differential metabolites were selected on the basis of the statistically significant threshold of VIP > 1 and P < 0.01 in the Student’s t test and FC > 2 or < 0.5. Then, the differential metabolites were validated through MetaboAnalyst and KEGG databases. Finally, 11 differential metabolites were screened in the serum (Table 2) and 8 differential metabolites were screened in the liver samples (Table 3) among the control, NCTDL, and NCTDH groups. Figure 8 illustrates the heat map of the changes in the differential metabolites of the control and NCTD-treated groups of the rat serum (Fig. 8A) and liver (Fig. 8B). From the heat map analysis, most of the metabolites in rat serum were up-regulated, while the metabolites in liver almost showed a downward trend.
Pathway analysis
Eleven differential metabolites were found in the rat serum samples, and all of these biomarkers were imported into MetaboAnalyst for pathway analysis. Five pathways were found related to the hepatotoxicity induced by NCTD in rat serum (Fig. 9A). Eight differential metabolites were found in liver tissue samples, and five pathways also showed closely relation to NCTD-induced hepatotoxicity (Fig. 9B). Among them, four metabolic pathways with high pathway impact values including the sphingolipid metabolism, purine metabolism, arachidonic acid metabolism, and glycerophospholipid metabolism were considered as key metabolic pathways related to NCTD hepatotoxicity. Moreover, a comprehensive metabolic network in rat serum and liver associated with NCTD-induced hepatotoxicity (Fig. 10) was established in our study based on the KEGG pathway database.
Pathway analyses of metabolites in rat serum and liver. A Pathway analysis of metabolites in rat serum. 1: Sphingolipid metabolism; 2:Purine metabolism; 3: Glutathione metabolism; 4: Glycerophospholipid metabolism; 5: Tryptophan metabolism. B Pathway analysis of metabolites in rat liver. 1: Arachidonic acid metabolism; 2: Glycerophospholipid metabolism; 3: Pentose and glucuronate interconversions; 4: Amino sugar and nucleotide sugar metabolism; 5: Starch and sucrose metabolism
Discussion
This paper studied the serum and liver tissue samples of rats exposed to NCTD by combining the traditional hepatotoxic evaluation indices and metabolomics technology. The biochemical results showed that the levels of ALT and TBIL in rat serum were significantly increased after administration of NCTD. These results were similar to those of previous reports (Fan et al. 2017; Li et al 2017), indicating that NCTD caused damage to hepatocytes.
Simultaneously, histopathological changes and ultrastructure analysis also confirmed injuries to the liver. Ultrastructure analysis showed that the endoplasmic reticulum was dilated, the mitochondria were ballooned, and several vacuoles were present after NCTD treatment, indicated that NCTD high dose caused mild centrilobular hepatocyte hypertrophy and hepatocyte swelling in rat liver.
The metabolites of NCTD-induced liver injury in rats were identified by UPLC/Q-TOF–MS metabolomics. A total of 19 differential metabolites were detected in the serum and liver at negative and positive modes after NCTD treatment. These differential metabolites primarily involved nine metabolic pathways associated with NCTD hepatotoxicity, including sphingolipid metabolism, purine metabolism, glutathione metabolism, glycerophospholipid metabolism, tryptophan metabolism, arachidonic acid metabolism, pentose and glucuronate interconversions, amino sugar and nucleotide sugar metabolism, and starch and sucrose metabolism. In this study, four metabolic pathways with high pathway impact values in serum and liver were discussed, respectively.
This study found that sphingolipid metabolism was perturbed in the serum. Sphingolipids are key molecules involved in cell growth, differentiation, and programmed death. Sphingolipid metabolism is directly or indirectly related to the abnormal status of many diseases and toxic responses to exogenous compounds in vivo. Studies indicated that sphingolipid metabolism is a key biological network intimately involved in hepatocyte toxicity (Li et al. 2020; Shi et al. 2021).
It has been reported that sphinganine-1-phosphate plays an important role in hepatotoxic metabolic disorders induced by Tripterygium wilfordii Hook F. Likewise, the content of sphinganine-1-phosphate in rat serum was increased, which indicated that cell death was induced via the production of reactive oxygen species and activation of cell factors related to cell apoptosis (Qu et al. 2015). Furthermore, oxidized glutathione, the oxidation products of glutathione, is a good indicator of oxidative stress (Asensi et al. 1999; Dalle-Donne et al. 2006). The content of oxidized glutathione increased in rat serum in this study, we speculate that sphingonine-1-phosphate could transformed into oxidized glutathione by the results of metabolic pathway and then caused rat liver injury through oxidative stress.
To sum up, the sphingolipid metabolic pathway is considered to be closely related to NCTD-induced hepatotoxicity, and the increase of sphinganine-1-phosphate in the serum is involved in the process of NCTD-induced hepatotoxicity.
Xanthosine participates in purine metabolism and it is one of the important intermediate metabolites of purine metabolism, which plays an important role in gene synthesis, and metabolism regulation. A recent report pointed out that xanthosine was closely related to the development of metabolic diseases (Xiao et al. 2019). Moreover, a recent study indicated that the increase in xanthosine levels was one of the potential metabolic markers for the differential diagnosis of chronic hepatitis B and hepatocellular carcinoma (Zhang et al. 2019). Furthermore, oxidative stress is closely linked to the disorder of purine metabolism (Guan et al. 2021). In this study, the level of xanthosine in NCTD-treated groups was significantly increased, suggesting that the increase in xanthosine led to oxidative stress.
As a precursor of arachidonic acid metabolism, prostaglandin E2 is a major regulator of the immune response, and it implicated in the induction of inflammatory symptoms (Yuhas et al. 2007). A recent study has proposed that prostaglandin E2 exhibits protective effects against multiple types of liver injury, and that hepatic prostaglandin E2 plays an important role in the protection against liver injury (Shimada et al. 2020). Moreover, researchers have pointed out that prostaglandins, including prostaglandin E2, prostacyclin, and misoprostol (the analog of prostaglandin E1) were found to be effective against kidney injuries (Paller and Manivel 1992). In our study, the liver of rats was damaged due to the administration of NCTD. Furthermore, the content of prostaglandin E2 was decreased, suggesting that hepatotoxicity induced by NCTD was related to arachidonic acid metabolism. Further research is needed to determine whether P2 has a protective effect on hepatotoxicity induced by NCTD in rats.
The major glycerophospholipids in the cell include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine, phosphatidylinositol, phosphatidic acid, phosphatidylglycerol, and cardiolipin. Among them, PC and PE are the most abundant phospholipids. PE is an essential substance for membrane integrity, cell division, maximum mitochondrial respiratory function, and membrane protein topology, and it is an important precursor for PC (Calzada et al. 2015). The decrease in PE and PC levels influence the integrity of the liver cell and mitochondrial membrane, affect cell growth and apoptosis (Chen and Sun 2019; Shi et al. 2021). In this study, PC and PE levels were decreased in the rat liver, which indicated that NCTD induces hepatotoxicity in rats by accelerating apoptosis in liver cells, thus destroying the cell structure.
UPLC/Q-TOF–MS metabolomics techniques combined with traditional methods were used to study the potential mechanism of NCTD-induced rat hepatotoxicity. First, the biochemical indicators and pathological changes in the liver tissue samples were significantly changed, suggested that NCTD-induced rat hepatotoxicity with the centrilobular hepatocyte hypertrophy, hepatocyte swelling and necrosis. These results proved that NCTD damaged rat liver. The result of metabolic analysis also showed alterations in 19 metabolites following NCTD administration. Furthermore, these metabolites were finally identified to have great relations to sphingolipid metabolism, purine metabolism, glycerophospholipid metabolism, and arachidonic acid metabolism. In conclusion, NCTD caused hepatotoxicity in rats and the toxicological mechanism might be related to oxidative stress and apoptosis, which provided a reference for elucidating the mechanism of NCTD-induced hepatotoxicity.
References
Asensi M et al (1999) Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Method Enzymol 299:267–276
Calzada E et al (2015) Phosphatidylethanolamine metabolism in health and disease. Int Rev Cel Mol Bio 321:29–38
Chen AWG et al (2018) Norcantharidin induce apoptosis in human nasopharyngeal carcinoma through caspase and mitochondrial pathway. Environ Toxicol 33(3):343–350
Chen X, Sun CY (2019) Application progress on metabonomics in toxicity assessment and toxicologic studies of natural products. Chin Tradit Herbal Drugs 50(5):1244–1257
Chi JH et al (2019) Studies on anti-hepatocarcinoma effect, pharmacokinetics and tissue distribution of carboxymethyl chitosan based norcantharidin conjugates. Carbohyd Polym 226(4):1–10
Dalle-Donne I et al (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52(4):601–623. https://doi.org/10.1373/clinchem.2005.061408
Ding XY et al (2012) Pharmacokinetics, tissue distribution, and metabolites of a polyvinylpyrrolidone-coated norcantharidin chitosan nanoparticle formulation in rats and mice, using LC-MS/MS. Int J Nanomed 7:1723–1735
Fan XT et al (2017) Toxicity of cantharidin and norcantharidin in Mice. Chin J Exp Tradit Med Form 23(15):118–123
Gao B et al (2021) Intratumoral administration of thermosensitive hydrogel co-loaded with norcantharidin nanoparticles and doxorubicin for the treatment of hepatocellular carcinoma. Int J Nanomed 16:4073–4085
Godzien J, Alonso-Herranz V, Barbas C, Armitage EG (2014) Controlling the quality of metabolomics data: new strategies to get the best out of the QC sample. Metabolomics 11(3):518–528
Guan T et al (2021) Metabolomics analysis of the effects of quercetin on renal toxicity induced by cadmium exposure in rats. Biometals 34(1):33–48
Jiang ZW, Chi JH, Han BQ, Liu WS (2017) Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohyd Polym 174:282–290
Katarey D, Verma S (2016) Drug-induced liver injury. Clin Med (Lond) 16(6):104–109
Li YN et al (2017) Study on chronic toxicity of chitosan-based norcantharidin sustained-release drug delivery system. Chin J Mar Drugs 36(5):33–41
Li LH, Wang HB, Jones JW (2020) Sphingolipid metabolism as a marker of hepatotoxicity in drug-induced liver injury. Prostag Oth Lipid M 151:106484
Lin X et al (2012) Preclinical evaluations of norcantharidin-loaded intravenous lipid microspheres with low toxicity. Expert Opin Drug Deliv 9(12):1449–1462
Liu XL et al (2017) Asialoglycoprotein receptor-targeted liposomes loaded with a norcantharimide derivative for hepatocyte-selective targeting. Int J Pharm 520(1–2):98–110. https://doi.org/10.1016/j.ijpharm.2017.02.010
Losser MR, Payen D (1996) Mechanisms of liver damage. Semin Liver Dis 16(4):357–367
Ma Q et al (2018) Unique responses of hepatocellular carcinoma and cholangiocarcinoma cell lines toward cantharidin and norcantharidin. J Cancer 9(12):2183–2190
McGill MR, Jaeschke H (2019) Biomarkers of drug-induced liver injury. Adv Pharmacol 85:221–239
Pachuta-Stec A, Nowak R, Pietrzak W, Pitucha M (2019) Synthesis and antioxidant activity of new norcantharidin analogs. Chem Biodivers 16(4):e1800673
Paller MS, Manivel JC (1992) Prostaglandins protect kidneys against ischemic and toxic injury by a cellular effect. Kidney Int 42(6):1345–1354
Pan MS, Cao J, Fan YZ (2020) Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin Med 15(55):1–28
Qu L et al (2015) Integrated targeted sphingolipidomics and transcriptomics reveal abnormal sphingolipid metabolism as a novel mechanism of the hepatotoxicity and nephrotoxicity of triptolide. J Ethnopharmacol 170:28–38
Rawat A et al (2016) 1H NMR-based serum metabolomics reveals erythromycin-induced liver toxicity in albino Wistar rats. J Pharm Bioallied Sc 8:327–334
Shi M et al (2021) Repeated low-dose exposures to sarin disrupted the homeostasis of phospholipid and sphingolipid metabolism in guinea pig hippocampus. Toxicol Lett 338:32–39
Shimada H et al (2020) The regulatory mechanism involved in the prostaglandin E2 disposition in carbon tetrachloride-induced liver injury. Prostag Leukotr Ess 155:1–8
Singh R et al (2021) Norcantharidin combined with 2-deoxy-d-glucose suppresses the hepatocellular carcinoma cells proliferation and migration. 3Biotech 11(3):142
Tawfike AF, Viegelmann C, Edrada-Ebel R (2013) Metabolomics and dereplication strategies in natural products. Methods Mol Biol 1055:227–244
Wang GF, Dong J, Deng LP (2018) Overview of cantharidin and its analogues. Curr Med Chem 25:2034–2044
Xiao Y et al (2019) A low ω6/ω3 ratio high-fat diet improves rat metabolism via purine and tryptophan metabolism in the intestinal tract, while reversed by inulin. J Agr Food Chem 67:7315–7324
Xu SY, Bian RL, Chen X (2003) Methodology of pharmacological experiment: People’s Health Publishing House, China
Yan Y et al (2020) UPLC/MS/MS-based metabolomics study of the hepatotoxicity and nephrotoxicity in rats induced by polygonum multiflorum thunb. ACS Omega 5:10489–10500
Yuhas Y, Azoulay-Alfaguter I, Berent E, Ashkenazi S (2007) Rifampin inhibits prostaglandin E2 production and arachidonic acid release in human alveolar epithelial cells. Antimicrob Agents Ch 51:4225–4230
Zhang AH, Sun H, Wang P, Han Y, Wang XJ (2012) Metabonomics for discovering biomarkers of hepatotoxicity and nephrotoxicity. Pharmazie 67:99–105
Zhang Y et al (2019) Quantification of serum purine metabolites for distinguishing patients with hepatitis B from hepatocellular carcinoma. Bioanalysis 11:1003–1013
Zhang JY et al (2020) Study on the mechanism of cantharidin-induced hepatotoxicity in rat using serum and liver metabolomics combined with conventional pathology methods. J Appl Toxicol 40:1259–1271
Zhou JB et al (2020) Norcantharidin: research advances in pharmaceutical activities and derivatives in recent years. Biomed Pharmacother 131:110755
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants no. 81760746, 81803838 and 82060754); Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302); Science and Technology Department of Guizhou province of China ([2016]2854, [2020]1Y376, [2019]1346, ZK[2021]532); Innovation talent team of Guizhou science and Technology Department (qiankehe platform talents [2020]5007); Education Department of Guizhou Province of China (GNYL[2017]006, [2017]078, [2012]311); Science and Technology Department of Zunyi city of Guizhou province of China ([2020]7, [2020]39, [2021]3); Science and Technology Department of Honghuagang District of Zunyi city of Guizhou province of China ([2020]17).
Funding
National Natural Science Foundation of China, 81803838.
Author information
Authors and Affiliations
Contributions
CD, JZ, LL and XL conceived and designed the experiment. WC, XW and QC performed the experiments. WC, XW and JZ analyzed the data. WC wrote the paper. All of the authors reviewed and approved the submitted version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
Author Weina Cheng declares that she has no conflict of interest. Author Qihong Chen declares that he has no conflict of interest. Author Xiaoning Wang declares that he has no conflict of interest. Author Liu Liu declares that she has no conflict of interest. Author Xiaofei Li declares that he has no conflict of interest. Author Cancan Duan declares that she has no conflict of interest. Author Jianyong Zhang declares that he has no conflict of interest.
Ethical approval
Animal protocols were approved by the Institutional Committee on Animal Care and Use of Zunyi Medical University (ZMUER2017-2-235).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, W., Chen, Q., Wang, X. et al. Illuminating the hepatotoxic mechanism of norcantharidin in rats using metabolomics analysis. Mol. Cell. Toxicol. 19, 551–564 (2023). https://doi.org/10.1007/s13273-022-00285-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-022-00285-3