Abstract
Objective
This manuscript reviews congenital anomalies and imaging findings associated with non-visualisation of the cavum septi pellucidi (CSP) found on prenatal sonogram.
Background
Observation of a normal cavum septi pellucidi (CSP) is an important landmark in the second and third trimester prenatal ultrasound evaluation of the fetal brain, and its visualisation provides reassurance of normal central forebrain development. Non-visualisation of the CSP is a prenatal sonographic finding, which in most cases is associated with neuroanatomical anomalies that include agenesis of the corpus callosum, schizencephaly, septo-optic dysplasia, holoprosencephaly, chronic hydrocephalus and acquired fetal brain injury. Isolated septal deficiency, a rare but controversial entity, is considered a variant of normal. Common pitfalls in the sonographic evaluation of CSP include columns of the fornix that mimic CSP, and prominent cavum vergae that can simulate non-visualisation of the CSP. When non-visualisation of the CSP is suspected, magnetic resonance imaging (MRI) of the fetal brain can confirm and evaluate associated anomalies.
Conclusion
Visualisation of the CSP is an integral component of the prenatal ultrasound and its non-visualisation is associated with other malformations, diagnosis of which is aided by MRI.
Teaching points
• Cavum septi pellucidi (CSP) is an important landmark in the prenatal ultrasound evaluation of the fetal brain, and is a marker for normal central forebrain development.
• Non-visualisation of the CSP is most commonly associated with other neuroanatomical abnormalities.
• Examination of the fetal brain by MRI can confirm the sonographic findings and evaluate for associated anomalies.
Similar content being viewed by others
Introduction
The cavum septi pellucidi (CSP) is an important landmark in the evaluation of the fetal neural axis, identified as a fluid-filled space between the leaves of the septum pellucidum. Visualisation of the CSP has become an essential component of a second and third trimester prenatal ultrasound [1, 2]. Correctly identifying its presence or absence is very important in predicting the course of fetal central forebrain development. CSP non-visualisation is rarely isolated; it is typically associated with malformations of prosencephalic development, which include agenesis/dysgenesis of corpus callosum (CC), schizencephaly, septo-optic dysplasia (SOD) and spectrum of holoprosencephaly (HPE), or non-visualisation of the CSP is secondary to a disruptive process caused by prolonged ischaemia, resulting in necrotic disruption of the septum pellucidum, as can be seen in severe chronic hydrocephalus, and acquired fetal brain injury. Barkovich et al. [3] have stressed the importance of absence of the septum pellucidum as a valuable clue in the diagnosis of these central nervous system (CNS) abnormalities. A step-wise algorithm based on post-natal magnetic resonance imaging (MRI) was described to facilitate the diagnostic process, which would be challenging to apply in prenatal ultrasound given technical and patient related limitations. Nevertheless, this finding should prompt detailed sonographic imaging with the assistance of fetal MRI to overcome the limitations posed by ultrasound.
This pictorial essay reviews the technical considerations, embryology, anatomy and spectrum of diseases associated with non-visualisation of the CSP imaged by prenatal sonography and MRI. Prognostic information is briefly addressed for the various diseases. Important sonographic pitfalls in the assessment of the CSP and helpful differentiating features amongst the neuroanatomical abnormalities will also be discussed.
Technical considerations
Sonography is the imaging technique of choice for prenatal screening of fetal abnormalities, given its widespread availability, low cost, and real-time evaluation of the fetus. High-frequency transducers, transvaginal sonography, dedicated 3D transducers have increased the ability of ultrasound to demonstrate structures or pathological conditions. Limitations inherent to sonography include operator dependency and skill, limited field of view and visualisation in the setting of maternal obesity, oligohydramnios and fetal head engagement. In particular, sonographic evaluation of the fetal CNS is limited by non-specific appearance of some anomalies, subtle parenchymal and migrational abnormalities that cannot be visualised, and advanced gestational age preventing evaluation of the posterior fossa, brain stem and entire brain. Consequently, sonographic findings may be inconclusive or insufficient to guide management. Fetal MRI has overcome many of the obstacles associated with sonography with the advent of ultrafast T2-weighted imaging, yielding images of high soft-tissue contrast and spatial resolution [4]. Fetal MRI is not limited by fetal position, an important factor for assessment of intracranial anatomy and maternal obesity. The multiplanar capability of MRI allows direct visualisation of intracranial anatomy in a manner not possible by sonography. Fetal MRI can confirm or exclude anomalies detected by sonography, and can demonstrate migrational and other disorders which may be difficult to appreciate sonographically. The additional information can be used to better counsel patients during pregnancy about the cause of CNS abnormality and outcome of the fetus. Fetal MRI is limited by fetal motion during image acquisition, which will degrade image quality, although patient fasting prior to the study would decrease the motion.
Embryology and normal anatomy
Development of the CSP and CC is closely associated because they are both derived from the commissural plate [5, 6]. At 12 weeks’ gestation, the CC begins development as a bundle of white matter tracts connecting the two cerebral hemispheres. Cavitation of the medial inferior commissural plate during CC formation gives rise to the two septa pellucida. Anatomically, the septa pellucida are thin, parallel vertical membranes that separate the frontal horns of the lateral ventricles. They are walled off by the CC superiorly and the fornix inferiorly. During fetal development, the septa pellucida are separated by a space filled with cerebrospinal fluid (CSF), termed the CSP. Posterior to the foramina of Monro, this space is termed the cavum vergae. Although commonly referred to as simply the CSP, the entire space is properly named the “cavum septi pellucidi et vergae”. The CSP is attached to the undersurface of the CC superiorly, to the anterior part of the fornix inferiorly and posterior to the reflected part of the CC (rostrum) anteriorly. The septa begin to fuse in an anterograde fashion at approximately 24 weeks’ gestation [1, 7]. In a sonographic study, CSP was seen in 100 % of normal fetuses between 18–37 weeks’ gestation and in 79 % of normal fetuses between 38–41 weeks’ gestation [8]. By term, complete posterior closure is seen in 97 % of infants so only the CSP is present and the cavum vergae is absent. By 6 months of age, the CSP is completely obliterated in 85 % of infants by the fusion of the septa pellucida to form a single septum pellucidum [9]. Visualisation of the CSP by 18–20 gestational weeks reassures proper development of the central forebrain [7, 8]. Routine ultrasound views include occipito-bregmatic and axial view through lateral ventricles and thalami. With cephalic presentation, transvaginal ultrasound assesses the CSP in the coronal plane. The CSP is well-visualised by MRI (Fig. 1) so that if abnormalities are detected, fetal MRI can confirm the finding and to search for associated anomalies which may be too subtle or difficult to identify by prenatal ultrasound. Li et al. [10] retrospectively evaluated 23 fetuses with septal leaflet abnormalities, and found that fetal MRI detected septal abnormalities in 23/23 patients and prenatal ultrasound in only 16/23 patients with a mean gestational age of diagnosis at 26 weeks (range 20–38 weeks). Additional central nervous system (CNS) findings were seen on MRI in 52 % of fetuses with better interobserver agreement among MRI readers than ultrasound readers. There is currently no consensus on appropriate timing of fetal MRI following non-visualisation of the CSP by ultrasound, although the majority of cases were detected in the late second trimester. Furthermore, this decision will be multifactorial taking into account diagnostic considerations at the time of prenatal ultrasound, management plan and prenatal counselling.
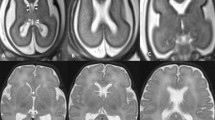
Normal anatomy of CSP in utero. a, b Occipito-bregmatic and coronal transvaginal ultrasound scans at 21 weeks’ gestation demonstrate normal appearance of CSP (arrow) and CC (dotted arrow). CSP should be seen by 18–20 weeks’ gestation. c, d Axial and coronal T2-weighted MRI at 33 weeks’ gestation demonstrate normal appearance of CSP (arrow) and CC (dotted arrow)
Anomalies
Absence of a fluid-filled CSP can result from primary prosencephalic maldevelopment or a secondary process with resultant destruction of the septal leaflets. It is associated with a range of disorders: agenesis/dysgenesis of the CC, schizencephaly, SOD, HPE, severe chronic hydrocephalus and acquired fetal brain injury. Rarely, agenesis of the CSP can be isolated.
Agenesis/dysgenesis of the corpus callosum
The CC is the largest bundle of white matter fibres connecting the two cerebral hemispheres and allows for intercommunication. The CC develops between 12 and 18 weeks of gestation from the lamina terminalis. Development of the CC is closely related to that of the CSP, both anatomically and embryologically [9]. Agenesis of the CC (ACC) is a common CNS anomaly with an incidence of 0.5–7 in 10,000 live births. The widely accepted theory for the embryogenesis of the CC suggests that the genu of the CC develop first, then the anterior body, posterior body, followed by the splenium. The exception to the orderly anterio-posterior callosal development is the rostrum, which forms last, typically by 20 weeks. Dysgenesis of the CC can be described as a partial agenesis of the CC or hypoplasia of the CC. In partial agenesis of the CC, the splenium and rostrum are usually absent and there may be hypoplasia of the remaining CC, and in callosal hypoplasia there is under-development of the entire CC [6, 11–13]. Prenatal imaging findings for ACC in addition to non-visualisation of the CSP include colpocephaly (enlargement of the trigone and occiptal horns of the lateral ventricle), high riding third ventricle, parallel orientation of the lateral ventricles, steer horn configuration of the frontal horns of lateral ventricles, convergence of median sulci towards the third ventricle, interhemispheric cyst, and abnormal course or absence of the pericallosal artery (Fig. 2). However, in dysgenesis of the CC, the CSP is typically present but may be shortened. The outcome of prenatally detected ACC is dependent on the presence or absence of associated anomalies. Patients with isolated ACC have the most favourable outcomes, with the majority experiencing relatively normal motor and cognitive development [14]. However, in a recent study by Fratelli et al. [15], 70 % of cases with agenesis or dysgenesis of the CC were associated with other fetal structural or chromosomal abnormalities, and 36 % of infants with isolated ACC had postnatal developmental delay. A full assessment therefore includes karyotyping and fetal MRI to search for more subtle features of genetic syndromes.
ACC. a, b Axial ultrasound and MRI at 33 and 35 weeks’ gestation respectively demonstrates non-visualisation of the CSP, parallel orientation of lateral ventricles (arrows), and colpocephaly (dotted arrow). c Coronal T2-weighted MRI through the frontal horns demonstrates absence of the CSP, ACC with steer horn configuration of the frontal ventricles (arrow)
Schizencephaly
This is a rare CNS malformation involving the grey matter, characterised by a cleft lined by grey matter extending from the pial surface to the lateral ventricle [16]. Schizencephaly has an incidence of 1.5 in 100,000 live births [17]. The aetiology is thought to be a disruption of gray matter migration in early embryogenesis. Non-visualisation of the CSP is an imaging finding in two-thirds of schizencephaly cases and is usually associated with fronto-parietal clefts. Other imaging findings include ACC, polymicrogyria and heterotopia [16, 18]. There are two types of schizencephaly. Type I is the closed-lip form, in which the edge of the cleft is fused. Type II is the open-lip form, in which the cleft extends from the ventricle to the surface of the brain and the edge of the cleft is not fused (Fig. 3) [19]. In a recent large survey of a regional register in the United Kingdom, 33 % of antenatally diagnosed cases of schizencephaly were established by fetal MRI only, an important modality in the evaluation of the cleft lining and sulcation pattern. Additionally, the majority of cases were identified after 22 weeks gestational age. Depending on the size and appearance of the cleft, diagnosis of schizencephaly may be difficult during screening ultrasound and, when visualised, may not be confidently differentiated from HPE or porencephaly (intracerebral cavitation secondary to haemorrhage and ischaemia) [17]. Distinguishing these entities is important given their differing prognosis.
Open-lip schizencephaly. a Coronal ultrasound through the atria at 30 weeks’ gestation with non-visualisation of the CSP (not shown) demonstrates a supratentorial cyst (asterisk), with a differential diagnosis of porencephaly versus schizencephaly. b Coronal T2-weighted MRI at 32 weeks’ gestation demonstrates absence of the CSP and bilateral frontoparietal clefts lined by grey matter (arrow) extending from the pial surface to the lateral ventricle consistent with open-lip schizencephaly
The extent of postnatal impairment depends on the location and extent of the cleft, with cleft bilaterality being the major prognostic factor. Unilateral clefts tend to be associated with hemiparesis, whereas bilateral clefts predict quadriparesis [19]. Other common sequelae include intellectual disability, hypotonia and seizures.
Septo-optic dysplasia
SOD (De Morsier Syndrome) is a congenital malformation syndrome that includes non-visualisation of the CSP, optic nerve/chiasm hypoplasia and hypothalamic pituitary dysfunction. It is a rare disorder, with an incidence of 1 in 50,000 live births [20]. Optic nerve hypoplasia can result in varying degrees of visual impairment and can be either unilateral or bilateral. Patients with SOD may have deficiencies of anterior pituitary gland hormones, resulting in hyperprolactinemia, hypothyroidism, diabetes insipidus and dwarfism [21]. Children with SOD should have regular endocrinology follow-up as hormonal insufficiencies may progress over time. Additional prenatal imaging findings associated with SOD include optic nerve hypoplasia, communication between anterior horns of lateral ventricles with separated foremost portions, mild ventriculomegaly and squared shape of anterior horns on coronal image (“batwing” configuration) (Fig. 4). However, since optic nerve/chiasm pathologies cannot be adequately evaluated on imaging, diagnosis of SOD cannot be confidently made with imaging alone, often requiring post-natal neuro-opthalmic evaluation for final diagnosis.
SOD. a Coronal ultrasound through the frontal horns demonstrates non-visualisation of the CSP and “squared off” morphology of the frontal horns (asterisk) suggestive of SOD. Columns of fornix are inferior to the CSP (arrows). b Coronal T2-weighted MRI displays characteristic “batwing” configuration of the frontal horns with communication between the horns (asterisk). c Axial T2-weighted MRI at 32 weeks of gestation confirms the absence of CSP and colpocephaly. There is separation of the foremost portion of the anterior horns (arrows). No cortical dysplasia was present. Postnatal ophthalmological evaluation was consistent with optic nerve hypoplasia
SOD-plus is a syndrome with SOD and a spectrum of neurological anomalies. Schizencephaly is commonly associated with SOD-plus (Fig. 5). SOD is associated with visual impairment, endocrine dysfunction, and developmental and cognitive delay. Focal motor deficits found on clinical evaluation distinguish SOD-plus from isolated SOD [20, 22].
SOD-plus. Prenatal ultrasound revealed non-visualisation of CSP and large periventricular cyst. a, b Coronal T2-weighted MRI at 27 weeks’ gestation demonstrates open-lip schizencephaly (asterisk) in the left frontoparietal region. There is absence of CSP, communication between the frontal horns (arrows) and dysgenesis of CC with absence of body (dotted arrow)
Holoprosencephaly
HPE is a spectrum of anomalies characterised by failure of the prosencephalon to develop into two cerebral hemispheres during the 5th week of gestation; namely, a ventral induction disorder, leading to absence of the CSP. It has an incidence of less than 1 in 10,000 live and still births [23]. Depending on severity, affected fetuses may die in utero or be born alive with brain developmental defects and mid-facial deformities. The severity of the facial dysmorphism correlates with the degree of cerebral pathology in a majority of cases. HPE is traditionally grouped according to the severity of forebrain non-cleavage into three types: alobar, semi-lobar and lobar. A fourth type of HPE, midline interhemispheric variant or syntelencephaly has recently been described. The classification of HPE by prenatal ultrasound depends on the severity of the malformation, with alobar being conspicuous on ultrasound. A review of 104 children with HPE found that only 22 % of cases were diagnosed by antenatal ultrasound. Moreover, 50 % and 83 % of children with alobar and semilobar HPE, respectively, were not diagnosed until birth. Furthermore, the reported presence of HPE in fetuses diagnosed initially with hydrocephalus by ultrasound ranges from 19 to 30 % [24, 25]; it is important to differentiate between hydrocephalus and HPE because of differing prognosis, inheritance pattern and prenatal counselling. In alobar HPE, non-visualisation of CSP is eclipsed by the obvious associated sonographic findings; the forebrain fails to separate and there is a complete absence of the falx cerebri, interhemispheric fissure, third ventricle, neurohypophysis, olfactory tract, CSP and CC. The thalami are fused, and there is a crescentic monoventricle (Fig. 6). In semilobar HPE, the anterior brain fails to separate with absent frontal horns and CSP. The posterior horns and trigone are present together with the posterior falx and posterior interhemispheric fissure. The thalami may be partially or fully fused with absence of the third ventricle, with presence of a monoventricle. In lobar HPE, a milder form, the cerebral hemispheres are developed and separated with the exception of the rostral aspect of the frontal lobe, as non-visualisation of the CSP is often the only finding by ultrasound. The correct findings in lobar HPE may be more difficult to recognise by ultrasound, with definitive assessment offered by fetal MRI [26]; the anterior brain non-cleavage is less severe than in the semilobar form, with full development of the third ventricle and posterior half of the CC, variable fusion of the frontal horns and fused fornices [27]. The central fusion of the frontal horns of the lateral ventricle gives rise to a flat, squared roof, which can mimic SOD. The presence of fused fornices and rudimentary frontal horns helps to distinguish lobar HPE from SOD. Presence of a dorsal cyst is most commonly seen in the alobar type (92 %) compared with the semilobar (28 %) and lobar (9 %) types. Its presence is thought to correlate with the degree of thalamic non-separation [27].
Alobar HPE. a Coronal ultrasound at 21 weeks’ gestation demonstrates apparent fusion of thalami (arrow), and overlying supratentorial CSF-filled cavity without discernible cortex, although significant near-field reverberation artefact was present. Sonographic considerations included alobar HPE and hydranencephaly and patient chose to continue with pregnancy. b Coronal T2-weighted MRI at 31 weeks gestation confirms the presence of fused thalami and absent falx. c Axial T2-weighted MRI demonstrates absence of CSP and non-cleavage of the hemispheres. The falx and interhemispheric fissure are completely absent. The single crescent-shaped forebrain ventricle (arrow), monoventricle (“Amadeus ventricle”), openly communicates with a large dorsal cyst (asterisk). d Sagittal T2-weighted MRI demonstrates communication of the monoventricle (arrow) with a large dorsal cyst (asterisk)
There is a wide phenotypic variance for infants with HPE. The severe forms are incompatible with life, and genetic counselling for families is recommended. In milder forms of HPE, a medical and genetic workup is recommended as associated conditions are common, including movement disorders, epilepsy and hypopituitarism. Chromosomal analysis is also useful for prognosis. In a population-based case–control study, the survival rate beyond 1 year of age was decreased to 2 % in patients with cytogenetic abnormalities, compared with 30–54 % survival rate among patients without cytogenetic abnormalities [28].
Severe chronic hydrocephalus
Hydrocephalus results from an imbalance between inflow and outflow of intracranial CSF. It is caused by overproduction, decreased absorption or obstruction of the CSF. The most common aetiologies for severe chronic hydrocephalus are aqueductal stenosis and Chiari malformation. Chronic hydrocephalus and raised intraventricular pressure can result in mechanical necrosis and disruption of the CSP [12]. Aqueductal stenosis can be primary or acquired. Primary causes include congenital narrowing of the aqueduct of Sylvius that accounts for 10 % of all causes of hydrocephalus (Fig. 7) [29], aqueductal forking or the presence of a septum within the aqueduct of Sylvius. Some cases may be inherited as an X-linked recessive trait. Acquired stenosis occurs as a sequela of inflammation or haemorrhage due to intrauterine infection, such as rubella, cytomegalovirus and toxoplasmosis. Infections can cause hydrocephalus by causing inflammation of the meninges and the ependymal lining of the ventricle, leading to impaired absorption of CSF or obstruction of the CSF flow through the ventricular system. In Chiari II malformation, downward herniation of the cerebellar hemispheres and/or vermis results when a normal-sized cerebellum develops in an abnormally small posterior fossa with a low tentorial attachment [30]. It is easily diagnosed by prenatal ultrasound and is often associated with ventriculomegaly, neural tube defects and agenesis/dysgenesis of the CC. It can be difficult to distinguish between severe chronic hydrocephalus, hydranencephaly and alobar HPE on prenatal ultrasound. Alobar HPE shows a monoventricle, complete non-cleavage of the cerebral hemispheres, complete absence of the falx cerebri, CC and CSP, and associated midline facial anomalies. Severe chronic hydrocephalus shows marked ventriculomegaly of the lateral and third ventricles, macrocephaly, normal cerebral cleavage, thinned cerebral cortex, normal falx cerebri and intact CC, but there is disruption of the CSP. Hydranencephaly shows absence of the cerebral cortex, which can be confirmed by fetal MRI and will be discussed in a later section.
Severe chronic hydrocephalus: aqueductal stenosis. a Occipito-bregmatic ultrasound view at 23 weeks demonstrates non-visualisation of the CSP and moderate but non-specific bilateral symmetric ventriculomegaly of the lateral ventricles. b Axial ultrasound at 29 weeks demonstrates progression in ventriculomegaly and macrocephaly. c Axial T2-weighted MRI at 30 weeks’ gestation demonstrates absent CSP, enlargement of the third ventricle (arrow) and bilateral lateral ventricles with colpocephaly (asterisk). d Sagittal T2-weighted MRI demonstrates lack of visualisation of a fluid-filled aqueduct of Sylvius (arrow), suspicious for stenosis. Postnatal MRI following decompression of the ventricular system confirmed aqueductal stenosis
Chiari malformation presents with a variety of symptoms, including dysphagia, upper extremity weakness, apneic spells and aspiration. Decompressing posterior fossa structures, closing any neural tube defect, and correcting hydrocephalus via shunt placement are the main interventions in post-natal surgical management [31].
Acquired fetal brain injury
Acquired fetal brain injury occurs when there is destruction of previously formed tissue. However, injury to structures necessary for normal brain development can also occur such that malformative and destructive lesions present simultaneously. Whether non-visualisation of the CSP is an associated finding depends on timing of the insult. There are numerous risk factors: underlying maternal disease, acute maternal disorders, infection, metabolic disease of the fetus, feto-fetal transfusion syndrome, iatrogenic causes and toxic agents. Prenatal ultrasound is an effective screening tool for porencephaly, haemorrhage and ventriculomegaly. However, fetal MRI is superior in showing acute and chronic manifestations of acquired brain lesions [32].
Hydranencephaly is the most severe form and is characterised by the complete or almost complete absence of cerebral cortex, which is replaced by fluid (Fig. 8). Hydranencephaly is a rare congenital anomaly occurring in less than 1 in 10,000 births. Non-visualisation of the CSP is an expected and obvious finding, overshadowed by the gross intracranial findings. It can be differentiated from HPE by identifying dural attachments and separate thalami on prenatal ultrasound [33]. While the aetiology is variable (infectious, toxic, iatrogenic, genetic, etc.), the likely pathogenic mechanism of hydranencephaly is early bilateral internal carotid occlusion with sparing of structures supplied by the vertebrobasilar system, including the brainstem, thalami, basal ganglia, cerebellum and portions of the temporal/occipital regions. On ultrasound, the characteristic necrotic brain tissue and blood may be visualised as a large, fluid-filled intracranial cavity with variable echogenicity [34]. If hydranencephaly occurs early in fetal development, the fetus may have microcephaly. Hydranencephaly can be confidently differentiated from severe chronic hydrocephalus by MRI, which can confirm the absence of cerebral cortex. Polyhydramnios may be present by the end of the second trimester because of poor fetal swallowing, but fetal movement is usually preserved. Prognosis is poor and neonates are neurologically devastated and rarely survive beyond infancy.
Hydranencephaly. a Coronal ultrasound at 20 weeks’ gestation demonstrates non-visualisation of CSP, and significant CSF-filled supratentorial cavity (asterisk). Anterior falx is faintly present (arrow), which excludes alobar HPE. b Axial T2-weighted MRI at 22 weeks’ gestation demonstrates extensive destruction of hemispheres. There is sparing of the cerebellum (c), midbrain (mid), thalami (t) and occipital lobes (occ), which are regions supplied by the vertebrobasilar artery. Cortical rim is absent excluding severe hydrocephalus and confirming hydranencephaly
Isolated agenesis of cavum septi pellucidi
Rarely, agenesis of the CSP can be isolated, with overall good prognosis, and although language delay and behavioural disorders with isolated agenesis of CSP have been described, the association has not been established with certainty (Fig. 9) [35, 36]. The diagnosis of isolated agenesis of CSP cannot be made with prenatal imaging, requiring postnatal neuro-ophthalmic and functional evaluation.
Isolated agenesis of CSP. Coronal T2-weighted MRI at 28 weeks’ gestation demonstrates “squared off” configuration and fusion of the anterior horns of the lateral ventricle, thus confirming absence of the CSP. The CC was normal (arrow), and no other anomaly was identified. The child had a normal neuro-opthalmic examination, with normal postnatal imaging
Pitfalls
A common pitfall is mistaking columns of the fornix for the CSP. Columns of the fornix are normal anatomic structures infero-posterior to and attaching to the CSP. It is composed of two bands, joined at the middle and separated at both ends. Although it is anatomically close to the CC and CSP, it is not related to them in embryonic development. Columns of the fornix can simulate the CSP because it is hypoechoic on ultrasound. It is important to distinguish this by identifying a centre line. Columns of the fornix show a linear reflection at their interface, therefore there are three echogenic lines on axial ultrasound view (Fig. 10). The CSP, on the other hand, appears as a fluid-filled space without a central line that is either triangular or rectangular in shape. Therefore, there are only two echogenic lines on axial ultrasound view [1]. Another pitfall occurs when a prominent CSP and cavum vergae displace CSP leaflets laterally. Normally the cavum vergae and CSP are contiguous in a mid-sagittal view. However, in some cases, displacement of the CSP leaflets laterally prohibits its identification sonographically, which can mimic non-visualisation of the CSP (Fig. 11).
Pseudo-presence of the CSP: columns of the fornix. Axial ultrasound through the fornix at 25 weeks’ gestation demonstrates its tri-linear configuration, which can be mistaken for a normal CSP. The lateral echogenic lines (solid arrows) result from the lateral margins of fornices while the central line (dotted arrow) represents the interface between the fornices
a, b, c Pseudo-absence of CSP: cavum vergae. a Coronal ultrasound through the frontal horns demonstrates non-visualisation of the CSP. The patient was referred for fetal MRI to assess for associated anomalies. b Coronal T2-weighted MRI demonstrates prominent CSP (asterisk) and lateral displacement of CSP leaflets mimicking absent CSP. c Axial T2-weighted MRI demonstrates extension of prominent CSP posterior to foramen of Monro consistent with cavum vergae
Algorithm
Non-visualisation of CSP should prompt a vigorous and detailed anatomical survey of midline structures, and a simplified algorithm is proposed that includes MRI evaluation to enable the radiologist to define and hopefully establish the diagnosis or at least offer a short differential diagnosis (Fig. 12). An algorithm initially proposed for postnatal evaluation of absence of the CSP can be adapted for prenatal imaging that encompasses both sonography and fetal MRI [3].
Simplified algorithm to assist in the diagnosis of intracranial pathology associated with absence of CSP. Adapted from [3]
Conclusion
The evaluation of the CSP is considered by many current guidelines as an integral part of prenatal sonogram. Visualisation of a normal CSP is a strong indicator of proper development of the forebrain Non-visualisation of the CSP can be a subtle sonographic finding, which in most cases is associated with a variety of anomalies as described in this pictorial essay. If an abnormality is suspected, MRI can confirm the finding and optimise evaluation of intracranial anatomy.
References
Callen PW, Callen AL, Glenn OA, Toi A (2008) Columns of the fornix, not to be mistaken for the cavum septi pellucidi on prenatal sonography. J Ultrasound Med 27:25–31
International Society of Ultrasound in Obstetrics & Gynecology Education Committee (2007) Sonographic examination of the fetal central nervous system: guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol 29:109–116
Barkovich AJ, Norman D (1989) Absence of the septum pellucidum: a useful sign in the diagnosis of congenital brain malformations. AJR Am J Roentgenol 152:353–360
Pugash D, Brugger PC, Bettelheim D, Prayer D (2008) Prenatal ultrasound and fetal MRI: the comparative value of each modality in prenatal diagnosis. Eur J Radiol 68:214–226
Griffiths PD, Batty R, Reeves MJ, Connolly DJ (2009) Imaging the corpus callosum, septum pellucidum and fornix in children: normal anatomy and variations of normality. Neuroradiology 51:337–345
Rakic P, Yakovlev PI (1968) Development of the corpus callosum and cavum septi in man. J Comp Neurol 132:45–72
Winter TC, Kennedy AM, Byrne J, Woodward PJ (2010) The cavum septi pellucidi: why is it important? J Ultrasound Med 29:427–444
Falco P, Gabrielli S, Visentin A, Perolo A, Pilu G, Bovicelli L (2000) Transabdominal sonography of the cavum septum pellucidum in normal fetuses in the second and third trimesters of pregnancy. Ultrasound Obstet Gynecol 16:549–553
Shaw CM, Alvord EC Jr (1969) Cava septi pellucidi et vergae: their normal and pathogical states. Brain 92:213–223
Li Y, Sansgiri RK, Estroff JA, Mehta TS, Poussaint TY, Robertson RL, Robson CD, Feldman HA, Barnewolt C, Levine D (2011) Outcome of fetuses with cerebral ventriculomegaly and septum pellucidum leaflet abnormalities. AJR Am J Roentgenol 196:W83–W92
Hetts SW, Sherr EH, Chao S, Gobuty S, Barkovich AJ (2006) Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. AJR Am J Roentgenol 187:1343–1348
Griffiths PD, Batty R, Connolly DA, Reeves MJ (2009) Effects of failed commissuration on the septum pellucidum and fornix: implications for fetal imaging. Neuroradiology 51:347–356
Ghi T, Carletti A, Contro E, Cera E, Falco P, Tagliavini G, Michelacci L, Tani G, Youssef A, Bonasoni P, Rizzo N, Pelusi G, Pilu G (2010) Prenatal diagnosis and outcome of partial agenesis and hypoplasia of the corpus callosum. Ultrasound Obstet Gynecol 35:35–41
Moutard ML, Kieffer V, Feingold J, Kieffer F, Lewin F, Adamsbaum C, Gélot A, Campistol I, Plana J, van Bogaert P, André M, Ponsot G (2003) Agenesis of corpus callosum: prenatal diagnosis and prognosis. Childs Nerv Syst 19:471–476
Fratelli N, Papageorghiou AT, Prefumo F, Bakalis S, Homfray T, Thilaganathan B (2007) Outcome of prenatally diagnosed agenesis of the corpus callosum. Prenat Diagn 27:512–517
Oh KY, Kennedy AM, Frias AE Jr, Byrne JL (2005) Fetal schizencephaly: pre- and postnatal imaging with a review of the clinical manifestations. Radiographics 25:647–657
Howe DT, Rankin J, Draper ES (2012) Schizencephaly prevalence, prenatal diagnosis and clues to etiology: a register-based study. Ultrasound Obstet Gynecol 39:75–82
Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Lévrier O, Figarella-Branger D, Girard N (2005) Assessment of cortical maturation with prenatal MRI: part II: abnormalities of cortical maturation. Eur Radiol 15:1781–1789
Denis D, Chateil JF, Brun M, Brissaud O, Lacombe D, Fontan D, Flurin V, Pedespan J (2000) Schizencephaly: clinical and imaging features in 30 infantile cases. Brain Dev 22:475–483
Barkovich AJ, Fram EK, Norman D (1989) Septo-optic dysplasia: MR imaging. Radiology 171:189–192
Traggiai C, Stanhope R (2002) Endocrinopathies associated with midline cerebral and cranial malformations. J Pediatr 140:252–255
Miller SP, Shevell MI, Patenaude Y, Poulin C, O’Gorman AM (2000) Septo-optic dysplasia plus: a spectrum of malformations of cortical development. Neurology 54:1701–1703
Orioli IM, Castilla EE (2010) Epidemiology of holoprosencephaly: prevalence and risk factors. Am J Med Genet C Semin Med Genet 154C:13–21
Nyberg DA, Mack LA, Bronstein A, Hirsch J, Pagon RA (1987) Holoprosencephaly: prenatal sonographic diagnosis. AJR Am J Roentgenol 149:1051–1058
Carrasco CR, Stierman ED, Harnsberger HR, Lee TG (1985) An algorithm for prenatal ultrasound diagnosis of congenital CNS abnormalities. J Ultrasound Med 4:163–168
Stashinko EE, Clegg NJ, Kammann HA, Sweet VT, Delgado MR, Hahn JS, Levey EB (2004) A retrospective survey of perinatal risk factors of 104 living children with holoprosencephaly. Am J Med Genet A 128A:114–119
Hahn JS, Barnes PD (2010) Neuroimaging advances in holoprosencephaly: Refining the spectrum of the midline malformation. Am J Med Genet C Semin Med Genet 154C:120–132
Croen LA, Shaw GM, Lammer EJ (2000) Risk factors for cytogenetically normal holoprosencephaly in California: a population-based case–control study. Am J Med Genet 90:320–325
Garne E, Loane M, Addor MC, Boyd PA, Barisic I, Dolk H (2010) Congenital hydrocephalus–prevalence, prenatal diagnosis and outcome of pregnancy in four European regions. Eur J Paediatr Neurol 14:150–155
McLone DG, Knepper PA (1989) The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 15:1–12
Sarnat HB (2008) Disorders of segmentation of the neural tube: Chiari malformations. Handb Clin Neurol 87:89–103
Prayer D, Brugger PC, Kasprian G, Witzani L, Helmer H, Dietrich W, Eppel W, Langer M (2006) MRI of fetal acquired brain lesions. Eur J Radiol 57:233–249
Lam YH, Tang MH (2000) Serial sonographic features of a fetus with hydranencephaly from 11 weeks to term. Ultrasound Obstet Gynecol 16:77–79
Sepulveda W, Cortes-Yepes H, Wong AE, Dezerega V, Corral E, Malinger G (2012) Prenatal sonography in hydranencephaly: findings during the early stages of disease. J Ultrasound Med 31:799–804
Malinger G, Lev D, Oren M, Lerman-Sagie T (2012) Non-visualization of the cavum septi pellucidi is not synonymous with agenesis of the corpus callosum. Ultrasound Obstet Gynecol 40:165–170
Damaj L, Bruneau B, Ferry M, Moutard ML, Garel C, Odent S, Adamsbaum C, Avni F, Tréguier C, Lazaro L (2010) Pediatric outcome of children with the prenatal diagnosis of isolated septal agenesis. Prenat Diagn 30:1143–1150
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hosseinzadeh, K., Luo, J., Borhani, A. et al. Non-visualisation of cavum septi pellucidi: implication in prenatal diagnosis?. Insights Imaging 4, 357–367 (2013). https://doi.org/10.1007/s13244-013-0244-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-013-0244-x