Abstract
Purpose
Fluid-structure interaction (FSI) models are more commonly applied in medical research as computational power is increasing. However, understanding the accuracy of FSI models is crucial, especially in the context of heart valve disease in patient-specific models. Therefore, this study aimed to create a multi-modal benchmarking data set for cardiac-inspired FSI models, based on clinically important parameters, such as the pressure, velocity, and valve opening, with an in vitro phantom setup.
Method
An in vitro setup was developed with a 3D-printed phantom mimicking the left heart, including a deforming mitral valve. A range of pulsatile flows were created with a computer-controlled motor-and-pump setup. Catheter pressure measurements, magnetic resonance imaging (MRI), and echocardiography (Echo) imaging were used to measure pressure and velocity in the domain. Furthermore, the valve opening was quantified based on cine MRI and Echo images.
Result
The experimental setup, with 0.5% cycle-to-cycle variation, was successfully built and six different flow cases were investigated. Higher velocity through the mitral valve was observed for increased cardiac output. The pressure difference across the valve also followed this trend. The flow in the phantom was qualitatively assessed by the velocity profile in the ventricle and by streamlines obtained from 4D phase-contrast MRI.
Conclusion
A multi-modal set of data for validation of FSI models has been created, based on parameters relevant for diagnosis of heart valve disease. All data is publicly available for future development of computational heart valve models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, fluid-structure interaction (FSI) simulations have become increasingly prevalent as computational resources have advanced, particularly in the field of medical research and heart valve disease. Patient-specific FSI simulations of heart valve disease can enable a better understanding of the valvular dynamics and complement traditional imaging modalities [1]. Despite their potential benefits, these simulation models have not yet been implemented in clinical practice, partly due to the lack of comprehensive validation [2]. Assessing the accuracy and reliability of FSI simulations is a complex task since it models both the fluid and structural domains [3], particularly in a clinical context where it is essential to determine the extent of their impact on diagnosis. Understanding the degree of accuracy of FSI simulations in a clinical setting is important to ensure reliable results that support medical decision-making. To establish the accuracy of simulations, validation against experimental data is a fundamental step to ensure reliable results, usable for clinicians [4].
Previous FSI simulation studies on heart valves have validated their model by comparing their simulation results to previous publications [5-8], to assess their agreement with other numeric data. While this approach is a common form of evaluation, it has limitations since the model accuracy is only put into relation to the accuracy of another model [4]. Only a few studies have developed a setup for experimental validation of FSI simulations [4, 9, 10]. Most commonly, parameters relevant to the development of numerical methods have been investigated [11-16], such as tissue strain and stress, vortex formation, and leaflet angular velocity. However, there is a lack of investigation into parameters relevant to diagnosis of heart valve disease, particularly the pressure gradient and the valve opening orifice dimensions.
Therefore, this study aimed to create experimental benchmarking data set for FSI simulation models, on an in vitro setup mimicking the left heart including a deforming mitral valve. A comprehensive multi-modal validation, with catheter pressure measurements, magnetic resonance imaging (MRI), and echocardiographic (Echo) imaging, was employed. This study aimed to measure and quantify key parameters such as ventricular and atrial pressure, velocity within the domain, and mitral valve opening to provide an extensive data set for future development and validation of FSI models. The data is provided openly for use by the research community [17].
Materials and Methods
In this study, an in vitro test setup is presented (Fig. 1), where a phantom mimicking the left heart including the mitral valve, was used to create benchmarking data for the validation of computer-based models for future heart valve simulations. Pulsatile physiological-inspired waveforms were produced with a computer-controlled motor and pump assembly. Catheter pressure measurements and medical imaging techniques were employed such that the pressure, velocity, and opening of the valve could be measured.
A schematic sketch of the pump setup within the MR scanner, with the 2 mT and 20 mT safety lines marked. The computer-controlled motor connected to a piston via a carbon fiber rod (grey arrow) is displayed along with sketches of the motor and pump configurations during the pump cycle causing the mitral valve (MV) to open and close. The piston pumps water in and out of the pump (white/black arrows), which in turn is connected to the phantom inside a plastic box, acting as a water reservoir. The blue arrows indicate the direction of the water
The In Vitro Test Setup
The positive displacement pump [18] has one inlet and one outlet, that allowed water to be pumped out of a membrane chamber and back in, as the piston in the pump moved back and forth (Fig. 1). The setup has a stroke volume of 0-130 ml, both as a forward- and backward flow, with stroke rates of 1-200 beats per minute, thus a large variety of cardiac outputs (CO) can be imitated. The pump was programmed to generate three different waveforms with flows corresponding to a CO of 2.9, 4.4, and 5.4 l/min, at 60 beats per minute.
The pump was driven by a rod-style actuator with a 56B10A motor (C type) (Myostat Motion Control Inc., Newmarket, ON Canada). It was operated with Cool Muscle Language coding implemented in the software Control Room (Myostat Motion Control Inc., Newmarket, ON Canada). To transfer the force from the motor to the pump, a carbon fiber rod connection was used. The distance between the components of the pump setup was calculated to ensure a proper position inside the MR room; with the MR-safe pump located in the bore close to the phantom, at the iso-center, and with the ferromagnetic motor positioned outside the 2 mT safety line (Fig. 1). The Echo exam and pressure measurements were performed at a different time point, outside the MR room.
The phantom was connected to the pump at the apex. It was mounted in a plastic box filled with 25 l of room temperature water (Fig. 1), which acted as a reservoir that allowed water to freely enter and exit the model.
Phantom Geometry and Flow Description
A simplified in vitro model of the left heart was developed to ensure a controllable experimental setup and reliable measurements with few confounding factors. The phantom included a ventricle, atrium, mitral and aortic valves, and the apex (Fig. 2a-b). The mitral valve leaflets were designed to open and close without the aid of chordae tendineae or papillary muscles, as for the native mitral valve, but purely driven by the water flow. In five out of six cases, this worked well. The aortic valve was modeled as a ball valve, instead of constructing a life-like aortic valve. This reduced the mechanical complexity, while still keeping its essential function of opening and closing during the pump cycle, thus reducing potential sources of malfunctioning during the experiments.
The flow entered and exited through the apex; when flow entered through the apex, the deformable mitral valve closed and the aortic valve opened. As the flow was reversed and exited through the apex, the mitral valve opened, and the aortic ball valve closed. Thus, diastolic and systolic valve configurations were obtained despite the rigid phantom heart model (Fig. 1). A uniform waveform of the flow at the apex was maintained with baffles at the base of the heart to ensure an evenly distributed flow through the phantom (Fig. 2b).
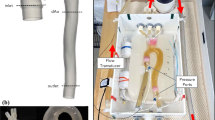
A schematic of the geometry of the phantom. (a) The computer-aided design (CAD) geometry used for 3D printing, illustrating how the atrium and mitral valve can be detached from the ventricle to change the silicone mitral valve and (b) how the mitral valve is securely fastened once the atrium is clamped into the ventricle. (c) The final 3D-printed phantom and the silicone mitral valve. (d) Some of the dimensions of the phantom and mitral valve. All exact measurements can be found in the CAD geometry files in the repository[17]
Furthermore, the phantom was equipped with flat surfaces for echo imaging, to ensure the same views were obtained for all investigated cases. The phantom also included two static pressure probes, reaching into the ventricle and atrium with a connection outside the model. Using Luer connections, catheters could be mounted to these for the pressure measurements (Fig. 2b). The dimensions of the phantom and the mitral valve approximate the dimensions of the human adult heart (Fig. 2d).
The phantom was 3D printed in a Form 2 printer (Formlabs Inc, Somerville, USA) with a stereolithography 3D printing technique in rigid (noncompliant) photopolymer resin (Grey Pro Resin, Formlabs Inc, Somerville, USA) (Fig. 2c). The rigid flow domain was printed in such a way that the atrium was detachable from the ventricle, which enabled the silicone mitral valve to be changed (Fig. 2a-b). The heart valves were cast in a clear flexible silicone material (Agilus30, Stratasys Ltd., Eden Prairie, USA) with stiffness Shore A25, A40, and A60 (Dreve Biopor AB, Damvig A/S, Copenhagen, Denmark). Tensile tests on the silicone material were performed on dog bone samples for future material modeling purposes, reported in Online Resource 1.
Measurements and Analysis
Pressure Catheters
The pressure in the atrium and ventricle was measured (Fig. 3) with fluid-filled catheters connected to a PowerLab Data Acquisition system (LabChart 7, ADInstruments, Dunedin, New Zealand). The catheters were connected to the pitot tube-like pressure probes of the phantom via Luer connections (Fig. 2) and the pressure at the atrium and ventricle was simultaneously recorded during 17 consecutive pump cycles. The pressure data was averaged over all cycles to reduce noise and cycle-to-cycle variations in the data.
Magnetic Resonance Imaging
The mass flow and bulk velocity were measured at the apex, the aortic valve, and in the atrium (Fig. 3) with 2D phase-contrast (PC) MRI at selected measurement planes and analyzed (Segment v3.3 R10057, https://medviso.com/segment/) [19] on a 1.5 T MR clinical scanner (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany). With 4D (3D + time) PC-MRI the mass flow and velocity were also recorded in the entire domain and analyzed (CAAS MR Solutions 5.2, Pie Medical Imaging BV, Maastricht, The Netherlands) to measure the flow through the same cross-sectional planes as with the 2D PC-MR for comparison. The bulk velocity through the mitral valve (Fig. 3) was also quantified as well as the velocity profile in the ventricle. Additionally, the flow through the phantom was visualized with streamlines based on the 4D PC-MRI data. The valve dynamics were imaged with cine MRI on which the valve opening, defined as the distance between the leaflet tips, was measured during peak flow (Online resource 1, Fig. S.2). All measurements were conducted by two observers, repeated 10 times, and averaged. First-order background phase correction was performed for all PC-MRI images [20, 21] and the MRI sequence parameters employed are detailed in Online resource 1, Table S.1.
Echocardiography
Commercially available ultrasound systems EPIQ CVx (Philips Medical Systems, Andover, MA, USA) and Vivid E95 (GE Healthcare; Vingmed Ultrasound, Horten, Norway) were used for both 2D and 3D imaging. The velocity through the valve was quantified with continuous wave Doppler Echo on the equivalent to a 3-chamber-view (IntelliSpace Cardiovascular 5, release 5.2, Philips Medical Systems, Best, The Netherlands). Both Echo systems showed equal image quality for velocity time integral (VTI) measurements. However, in the cases with the lowest CO, the spectral signal from the GE system was too weak, thus data from the Philips system was used. The GE system data was averaged over three pump cycles, while the Philips system data was averaged over two pump cycles. The temporal mean and maximum velocities were determined with the VTI based on the spectral velocity curve. The corresponding mean and maximum pressure difference (referred to as the pressure gradient in the clinical setting) was manually calculated with the Bernoulli equation using the density for water at room temperature (998 kg/m3). The software in the Echo systems were not used to determine the pressure difference as these use the density of blood in the Bernoulli equation.
The valve opening, defined as the distance between the leaflet tips, was measured on 2D 3-chamber-view Echo images from the GE system (Online resource 1, Fig. S.2 ). The opening area was measured on 3D images from the Philips system (4D MV assessment, TOMTEC Imaging Systems GmbH, Unterschleissheim, Germany). For the case with the medium stiff valve and the lowest CO, data from the GE machine was used. All manual Echo measurements were conducted 10 times and averaged, performed by two observers.
An overview of parameters measured during the phantom experiments. Red (numbers 1–2) marks the locations where the internal pressure was measured, green (number 3) marks where the Doppler measurements were performed, and purple (numbers 4–8) indicates where the MRI measurement planes were positioned. Here, the abbreviations AV and MV represent the aortic valve and the mitral valve
Investigated Flow Cases
Six different in vitro tests were investigated (Table 1). The cases are named accordingly: “Soft”, “Medium”, and “Hard” refer to the valve stiffness and 2.9, 4.4, and 5.4 refer to the CO in l/min.
For each flow case, all measurements described in the previous section were conducted and are freely available at the Zenodo repository with the assigned digital object identifier DOI: https://doi.org/10.5281/zenodo.10117609 [17]. The STL and STEP files of the phantom geometry, used for 3D printing, are also available.
Results
For visual assessment, snapshots from full-cycle videos obtained from cine MRI, 2D and 3D Echo are depicted to visualize the valve dynamics (Fig. 4). The full-cycle videos of cine MRI and 2D Echo imaging of the case Hard-4.4 are found in Online Resources 2 and 3.
The maximum mitral valve (MV) opening and complete closure imaged with cine MRI, 2D, and 3D Echo, is shown for case Hard-4.4 to exemplify the results. The white arrows mark the leaflet opening and closure in the 3D Echo images and the valve sketches represent the orientation of the valve for each modality
Validation of Experimental Setup
Technical validation of the experimental setup was performed to ensure reliable results. The 2D PC-MRI measurements were conducted at the aortic valve and the atrium of the phantom at the start and the end of each MRI exam of each flow case. A comparison of these two measurements showed a difference of 0.45 ± 0.35 l/min (3.5%) and 0.86 ± 0.67 l/min (6.7%) at the aortic valve and atrium, respectively. Pressure measurements in the ventricle were conducted over 17 consecutive pump cycles. A comparison showed a cycle-to-cycle variation of 0.5% for all cases, except for case Soft-2.9 which exhibited a 4.3% variation.
Catheter Pressure Measurements
The pressure measurements showed similar flow behavior in all six cases (Online Recourse 1, Fig. S.4). Therefore, only case Medium-4.4 is shown, to exemplify the results (Fig. 5). For the ventricular pressure, differences in amplitude were noted, where a higher peak pressure was observed for higher CO. The atrium was open to the surrounding water reservoir, thus nearly zero (atmospheric) pressure was recorded. The raw data, for all flow cases, is available in the data repository [17].
MRI Measurements
Velocity measurements at the apex, atrium, and aortic valve showed similar behavioral results for all six flow cases both for 2D and 4D PC-MRI measurements. A higher peak velocity was observed for the cases with higher CO; to exemplify the results, case Medium-4.4 is shown (Fig. 6). The remaining measurements are illustrated in Online Resource 1, Fig. S.5, and reported in the repository [17]. The flow velocity through the mitral valve increased with increasing CO, measured with 4D PC-MRI data (Fig. 6). A higher peak flow was also observed for increased valve stiffness due to a narrower opening which accelerated the flow.
In the 4D PC-MRI data, the field of view did not cover the entire phantom, leaving the end of the apex outside the measurable domain. Therefore, the circular cross-sectional plane for flow measurements at the apex was positioned across the baffles of the phantom instead. This resulted in a larger cross-sectional area for the 4D PC-MRI measurement plane compared to the corresponding 2D PC-MRI plane, thus, lower velocities were observed in the 4D PC-MRI apex measurements.
In case Soft-4.4, the softest valve could not withstand the high pressure at CO = 4.4 l/min, which caused it to prolapse (videos of the cine MRI and 2D Echo imaging can be found in Online Resources 4 and 5). To not damage the silicone valve, the 4D PC-MRI sequence was not conducted for this case.
The velocity through cross-sectional planes located at the apex, atrium, aortic valve, and mitral valve, measured with both 2D PC-MRI and 4D PC-MRI, shown for case Medium-4.4. The measurements were spatially averaged across the region of interest, thus the average velocity (dark pink and yellow) is reported together with the standard deviation (light pink and yellow shade) of the measurements. The sketches of the phantom show where the individual measurements were taken, note that the position of the apex measurements differs between the 2D and 4D PC-MRI planes
Velocity profiles were obtained from 4D PC-MRI data along the central lines in y- and z-direction in a cross-sectional plane of the ventricle (Fig. 7c). Figure 7a shows the data for case Medium-4.4, the other cases are available in the data repository [17] and visualized in the Online Recourse 1 (Fig. S.6). With the mitral valve closed, a low and uniform velocity profile is observed. In the latter half of the pump cycle, a distinct jet through the mitral valve is observed, resulting in recirculation areas. In the velocity profile along the y-direction, a slight dent is present at the center, attributed to the ventricular pressure probe located 3.6 mm downstream. The velocity amplitude peaks at 0.75 s, closely correlated with the pulsatile inlet condition.
Streamlines generated from 4D PC-MRI data (Fig. 7d-e) visualize the flow behavior. With the closed mitral valve, water exits through the aortic valve, accelerating through the ventricular outflow tract. At the narrowest part of the open mitral valve, the highest velocity is observed, later decelerated by the baffles at the apex. Recirculation zones within the ventricle are visible, as indicated by the velocity profile.
Visual assessment of the flow in the domain, for case Medium-4.4. (a) The velocity profile along lines in the y- and z-direction in the ventricle. The y-axes in the plots indicate the position along the measurement lines. (b) The graph indicates where in the pump cycle the time points are chosen and (c) shows the position of the measurement within the phantom. The streamlines are shown to visualize the flow inside the phantom for (d) the closure (at t = 0.25 s) and (e) the peak opening (at t = 0.75 s) of the mitral valve (MV)
The valve opening distance was measured on cine MRI data (Online Resource 1, Fig. S.2). Generally, a larger opening was seen for increased CO and decreased valve stiffness, however, this trend was not seen for cases Soft-2.9 and Medium-2.9. The valve opening measurements varied between 5 mm and 6.1 mm with a standard deviation corresponding to 2.8–5.1% (Table 2). The cine MRI measurements showed an interobserver variability of 6.9 ± 4.9%.
Echo Measurements
Doppler Echo velocity measurements through the mitral valve show increased peak velocity for increased CO and stiffness of the valve, due to a smaller opening orifice (Fig. 8). The maximum and mean velocity and pressure difference through the mitral valve were determined from the VTI and the Bernoulli equation (Table 3). The Echo measurements showed an interobserver variability of 24 ± 13%.
For the valve opening distance measurements, ranging from 5.5 to 6.3 mm with an internal deviation of 2.4–5.1% (Table 4), an increased opening is observed for increased CO. Similarly, the valve opening area measurements of 0.52–0.69 cm2 with a variation of 7.9–14% (Table 4), increase for higher CO. Illustrations of how the measurements were conducted are reported in Online Resource 1, Fig. S.3.
Discussion
This study aimed to create a set of high-quality and reliable experimental data for the development and validation of cardiac-motivated FSI models, with a phantom setup mimicking the left heart. Pressure measurements, MRI flow measurements, and Echo imaging were used to quantify clinically important measurements typically used for diagnosis of heart valve disease. Specifically, the ventricular and atrial pressure, the velocity within the domain, and the mitral valve opening were quantified in this multi-modal study and are openly provided for the research community [17].
The final design of the phantom was driven by our intended future use, which involves conducting patient-specific mitral valve simulations. To create a highly controllable test environment, the phantom model was reduced to its final configuration to minimize confounding factors and ensure reliable measurements. The intention is to compare the phantom data to its corresponding future FSI simulation results; thus it can be misleading to compare the phantom to the human heart itself due to its simplified nature. One approach on how to implement a corresponding FSI simulation is presented by Christierson et al. [22]. However, the data provided in this study may be freely used as input parameters to the numeric model and to compare the simulation output to the remaining measurements.
Validation of Experimental Setup
A comparison of flow measurements at the start and end of each MRI exam exhibited a low cycle-to-cycle variation, which demonstrated that the setup was reliable over time, with a steady pulsatile flow. This agrees with preliminary experiments from another study on the same motor-and-pump setup, which showed a Bland-Altman bias of 1.8% and an overall MRI error of less than 5% compared to timer and beaker [23]. The cycle-to-cycle variation in the pressure measurements was smaller than that measured with 2D PC-MRI, which may be attributed to the longer measurement duration for MRI. The pressure was measured for 20 s, while the time between 2D PC-MRI scans at the start and end of the experiments was about 30–45 min.
Catheter Pressure Measurements
The transcatheter approach is considered the gold standard for cardiac pressure measurement [24, 25]. However, catheter placement variability is a common source of error [25]. In our setup, consistent catheter position was secured through fixed attachment points incorporated in the 3D-printed phantom across all measured flow cases. To achieve an in vitro test setup, with no malfunctioning valve can be challenging [26]. Previous studies show the challenge in prevention of valve leakage and ensuring reliable pressure measurements [9]. When we calculated the pressure difference across the valve, by subtracting the atrial pressure from the ventricular pressure, the results were comparable with what is reported in literature for valves of different stiffnesses and flow velocities [27].
MRI Measurements
We found discrepancies in the amplitude between 2D and 4D PC-MRI flow measurements. For the apex, this discrepancy is mainly attributed to differences in the position of measurement planes within the phantom, which resulted in different cross-sectional areas and thus deviating velocity readings. However, despite equal positions of the measurement planes at the atrium and aortic valve, differences in velocity measurements were still observed. It has been shown that 4D PC-MRI data may underestimate peak velocities [28, 29], which may account for the observed differences. Additionally, the spatial standard deviation in 4D PC-MRI measurements is larger than in the 2D PC-MRI data, thus a higher variation in velocity in the 4D PC-MRI data was obtained. The lower contrast in 4D PC-MRI data compared to 2D PC-MRI data made accurate delineation of the region of interest for velocity measurements challenging. As the delineated area might include velocity measurements outside the actual flow domain, the average velocity decreases, resulting in an underestimation.
The 4D PC-MRI data allowed qualitative assessment of the ventricular flow during a typical pump cycle. However, the 1.5 mm voxel size in the 4D PC-MRI data could potentially provide insufficient resolution of the velocity jet, thus under-sampling the maximum velocity in the velocity profile, as reported in literature [18]. An alternative is to use particle imaging velocimetry (PIV) for quantification of the velocity profile. This offers higher spatial resolution [9] but requires a transparent flow domain and the material refractive index needs to be accounted for [30]. Despite the spatial resolution limitations, the velocity profile exhibited an expected behavior. Thus, the 4D PC-MRI data can provide a qualitative assessment of the flow behavior, which has the potential to support the development of future FSI models [31].
Echo Measurements
The overall trend and maximum amplitude measured with Doppler align with MRI flow measurements. A higher velocity through the valve is observed for increased CO and valve stiffness, an effect also seen for the mean and maximum pressure differences, consistent with the Bernoulli equation. However, a comparison of catheter pressure measurements and Doppler measurements shows Doppler tends to overestimate the maximum pressure difference across the valve, consistent with literature [32-34]. Despite this, Echo measurements are commonly used in the clinical setting, since it is a non-invasive and easily accessible method [35], which further highlights the importance of the conducted measurements in our study as they provide additional insights into Echo measurements.
Identification of the valve was generally easy in most acquired images, despite the potential challenges of imaging 3D-printed materials [36]. However, noisy images, particularly in case Hard-4.4, posed difficulties in accurately identifying valve leaflets and thus measuring distances, which is also reflected in the interobserver variability. Geometric measurements in Echo images are influenced by the angle of the probe relative to the object of interest. Angled cross-sectional planes risk skewed distances and inaccurate geometric measurements. Conversely, 3D volume measurements allow manual positioning of the measurement plane, to ensure proper alignment and avoid skewed distances [37]. In our data, the alignment of the measurement plane was not always perpendicular to the Echo beam itself, which resulted in varying lateral resolution and thus introduced uncertainty in area measurements despite proper alignment in relation to the valve. Nonetheless, the measured opening area follows the same trend as the distance measurements, with larger openings for higher CO and smaller openings for higher valve stiffness.
Limitations
A limiting factor in this study is that prolapse of the valve was observed in the case Soft-2.9. The soft valve could not withstand the peak pressure that arose at CO 4.4 l/min. Thus, we do not recommend this case to be used for the validation of FSI simulations.
Conclusions
In this study, a phantom setup mimicking the left heart was developed and used to create benchmarking data that can be used for future development and validation of cardiac-inspired fluid-structure interaction (FSI) models. The setup showed good repeatability and a low cycle-to-cycle variation, thus providing reliable measurements. In the in vitro experiments, parameters relevant to the diagnosis of heart valve disease were measured and quantified by modalities considered the gold standard in clinical practice. Catheter measurements were used to record ventricular and atrial pressure. The velocity within the phantom was quantified with 2D and 4D (3D + time) PC-MRI measurements, while the valve opening was assessed on cine MRI images as well as 2D and 3D Echo images. Doppler Echo analysis was used to quantify the velocity through the mitral valve and the pressure difference across the valve. All obtained results, including the phantom model, have been openly published and made publicly available [17] to facilitate the validation and development of future FSI models for clinical cardiac applications.
References
N. A. Tenenholtz, P. E. Hammer, R. J. Schneider, N. V. Vasilyev, and R. D. Howe, ‘On the design of an interactive, patient-specific surgical simulator for mitral valve repair’, IEEE International Conference on Intelligent Robots and Systems, pp. 1327–1332, 2011, doi: https://doi.org/10.1109/IROS.2011.6048851.
J. P. Rabbah, N. Saikrishnan, and A. P. Yoganathan, ‘A novel left heart simulator for the multi-modality characterization of native mitral valve geometry and fluid mechanics’, Ann Biomed Eng, vol. 41, no. 2, pp. 305–315, 2013, doi: https://doi.org/10.1007/s10439-012-0651-z.
G. Marom, ‘Numerical Methods for Fluid–Structure Interaction Models of Aortic Valves’, Archives of Computational Methods in Engineering, vol. 22, no. 4, pp. 595–620, 2015, doi: https://doi.org/10.1007/s11831-014-9133-9.
S. Schoenborn, S. Pirola, M. A. Woodruff, and M. C. Allenby, ‘Fluid-Structure Interaction Within Models of Patient-Specific Arteries: Computational Simulations and Experimental Validations’, IEEE Rev Biomed Eng, 2022, doi: https://doi.org/10.1109/RBME.2022.3215678.
L. Feng et al., ‘On the chordae structure and dynamic behaviour of the mitral valve’, IMA Journal of Applied Mathematics (Institute of Mathematics and Its Applications), vol. 83, no. 6, pp. 1066–1091, 2018, doi: https://doi.org/10.1093/imamat/hxy035.
L. Cai et al., ‘Some Effects of Different Constitutive Laws on FSI Simulation for the Mitral Valve’, Sci Rep, vol. 9, no. 1, pp. 1–15, 2019, doi: https://doi.org/10.1038/s41598-019-49161-6.
D. Collia, L. Zovatto, and G. Pedrizzetti, ‘Analysis of mitral valve regurgitation by computational fluid dynamics’, APL Bioeng, vol. 3, no. 3, pp. 1–10, 2019, doi: https://doi.org/10.1063/1.5097245.
F. Xu et al., ‘Computational investigation of left ventricular hemodynamics following bioprosthetic aortic and mitral valve replacement’, Mech Res Commun, vol. 112, p. 103604, 2021, doi: https://doi.org/10.1016/j.mechrescom.2020.103604.
A. Hessenthaler, N. R. Gaddum, O. Holub, R. Sinkus, O. Röhrle, and D. Nordsletten, ‘Experiment for validation of fluid-structure interaction models and algorithms’, Int J Numer Method Biomed Eng, vol. 33, no. 9, Sep. 2017, doi: https://doi.org/10.1002/cnm.2848.
A. Kalmbach and M. Breuer, ‘Experimental PIV/V3V measurements of vortex-induced fluid-structure interaction in turbulent flow-A new benchmark FSI-PfS-2a’, J Fluids Struct, vol. 42, pp. 369–387, Oct. 2013, doi: https://doi.org/10.1016/j.jfluidstructs.2013.07.004.
F. Domenichini and G. Pedrizzetti, ‘Asymptotic Model of Fluid–Tissue Interaction for Mitral Valve Dynamics’, Cardiovasc Eng Technol, vol. 6, no. 2, pp. 95–104, 2015, doi: https://doi.org/10.1007/s13239-014-0201-y.
L. Feng, H. Gao, B. Griffith, S. Niederer, and X. Luo, ‘Analysis of a coupled fluid-structure interaction model of the left atrium and mitral valve’, Int J Numer Method Biomed Eng, vol. 35, no. 11, Nov. 2019, doi: https://doi.org/10.1002/cnm.3254.
H. Gao, X. Ma, N. Qi, C. Berry, B. E. Griffith, and X. Luo, ‘A finite strain nonlinear human mitral valve model with fluid-structure interaction’, Int J Numer Method Biomed Eng, vol. 30, pp. 1597–1613, 2014, doi: https://doi.org/10.1002/cnm.2691.
I. Fumagalli et al., ‘An image-based computational hemodynamics study of the Systolic Anterior Motion of the mitral valve’, Comput Biol Med, vol. 123, no. May, p. 103922, 2020, doi: https://doi.org/10.1016/j.compbiomed.2020.103922.
B. Biffi et al., ‘A workflow for patient-specific fluid–structure interaction analysis of the mitral valve: A proof of concept on a mitral regurgitation case’, Med Eng Phys, vol. 74, pp. 153–161, 2019, doi: https://doi.org/10.1016/j.medengphy.2019.09.020.
W. Mao, A. Caballero, R. McKay, C. Primiano, and W. Sun, ‘Fully-coupled fluid-structure interaction simulation of the aortic and mitral valves in a realistic 3D left ventricle model’, PLoS One, vol. 12, no. 9, pp. 1–22, 2017, doi: https://doi.org/10.1371/journal.pone.0184729.
L. Christierson et al., ‘Multi-modal phantom experiments, mimicking flow through the mitral heart valve [Data set]’, Zenodo. [Online]. Available: https://doi.org/10.5281/zenodo.10117609
J. Töger, S. Bidhult, J. Revstedt, M. Carlsson, H. Arheden, and E. Heiberg, ‘Independent validation of four-dimensional flow MR velocities and vortex ring volume using particle imaging velocimetry and planar laser-Induced fluorescence’, Magn Reson Med, vol. 75, no. 3, pp. 1064–1075, Mar. 2016, doi: https://doi.org/10.1002/mrm.25683.
E. Heiberg, J. Sjögren, M. Ugander, M. Carlsson, H. Engblom, and H. Arheden, ‘Design and validation of Segment-freely available software for cardiovascular image analysis’, BMC Med Imaging, vol. 10, no. 1, 2010.
P. Walker, G. Cranney, M. Scheidegger, G. Waseleski, G. Pohost, and A. Yoganathan, ‘Semiautomated method for noise reduction and background phase error correction in MR phase velocity data’, Journal of Magnetic Resonance Imaging, vol. 3, pp. 521–530, 1993. https://doi.org/10.1002/jmri.1880030315.
A. Chernobelsky, O. Shubayev, C. R. Comeau, and S. D. Wolff, ‘Baseline correction of phase contrast images improves quantification of blood flow in the great vessels’, Journal of Cardiovascular Magnetic Resonance, vol. 9, no. 4, pp. 681–685, Jul. 2007, doi: https://doi.org/10.1080/10976640601187588.
L. Christierson et al., ‘Validation of fluid-structure interaction simulations of the opening phase of phantom mitral heart valves under physiologically inspired conditions’, Comput Biol Med, vol. 171, Mar. 2024, doi: https://doi.org/10.1016/j.compbiomed.2024.108033.
T. Lala et al., ‘Validation of real-time phase contrast MRI with online compressed sensing reconstruction in phantom and patients’, ISMRM & ISMRT Annual Meeting & Exhibition, 2023.
E. Chung, G. Chen, B. Alexander, and M. Cannesson, ‘Non-invasive continuous blood pressure monitoring: A review of current applications’, Frontiers of Medicine in China, vol. 7, no. 1. Higher Education Press Limited Company, pp. 91–101, Mar. 01, 2013. doi: https://doi.org/10.1007/s11684-013-0239-5.
M. VanAuker, A. Hla, J. Meisner, and J. Strom, ‘Simultaneous Doppler/catheter measurements of pressure gradients in aortic valve disease: a correction to the Bernoulli equation based on velocity decay in the stenotic jet.’, J Heart Valve Dis, vol. 9, pp. 291–298, 2000.
J. Sigüenza et al., ‘Fluid-structure interaction of a pulsatile flow with an aortic valve model: A combined experimental and numerical study’, Int J Numer Method Biomed Eng, vol. 34, no. 4, Apr. 2018, doi: https://doi.org/10.1002/cnm.2945.
P. C. Wiener, A. Darwish, E. Friend, L. Kadem, and G. S. Pressman, ‘Energy loss associated with in-vitro modeling of mitral annular calcification’, PLoS One, vol. 16, no. 2 February, pp. 1–13, 2021, doi: https://doi.org/10.1371/journal.pone.0246701.
P. Sjöberg et al., ‘Comparison of 2D and 4D Flow MRI in Neonates Without General Anesthesia’, Journal of Magnetic Resonance Imaging, vol. 57, no. 1, pp. 71–82, Jan. 2023, doi: https://doi.org/10.1002/jmri.28303.
J. Bock et al., ‘Validation and reproducibility of cardiovascular 4D-flow MRI from two vendors using 2 × 2 parallel imaging acceleration in pulsatile flow phantom and in vivo with and without respiratory gating’, Acta radiol, vol. 60, no. 3, pp. 327–337, Mar. 2019, doi: https://doi.org/10.1177/0284185118784981.
V. Kanyanta, A. Ivankovic, and A. Karac, ‘Validation of a fluid-structure interaction numerical model for predicting flow transients in arteries’, J Biomech, vol. 42, no. 11, pp. 1705–1712, Aug. 2009, doi: https://doi.org/10.1016/j.jbiomech.2009.04.023.
N. Hussein et al., ‘Simulation of semilunar valve function: computer-aided design, 3D printing and flow assessment with MR’, 3D Print Med, vol. 6, no. 1, Dec. 2020, doi: https://doi.org/10.1186/s41205-020-0057-8.
C. Dockerill, H. Gill, J. F. Fernandes, A. Q. X. Nio, R. Rajani, and P. Lamata, ‘Blood speckle imaging compared with conventional Doppler ultrasound for transvalvular pressure drop estimation in an aortic flow phantom’, Cardiovasc Ultrasound, vol. 20, no. 1, Dec. 2022, doi: https://doi.org/10.1186/s12947-022-00286-1.
T. A. Herrmann et al., ‘In vitro comparison of doppler and catheter-measured pressure gradients in 3D models of mitral valve calcification’, J Biomech Eng, vol. 135, no. 9, 2013, doi: https://doi.org/10.1115/1.4024579.
F. Donati et al., ‘Beyond Bernoulli: Improving the Accuracy and Precision of Noninvasive Estimation of Peak Pressure Drops’, Circ Cardiovasc Imaging, vol. 10, no. 1, Jan. 2017, doi: https://doi.org/10.1161/CIRCIMAGING.116.005207.
S. F. Nagueh et al., ‘Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography’, Journal of the American Society of Echocardiography, vol. 22, no. 2, pp. 107–133, Feb. 2009, doi: https://doi.org/10.1016/j.echo.2008.11.023.
S. Wang et al., ‘Manufacturing of Ultrasound- and MRI-Compatible Aortic Valves Using 3D Printing for Analysis and Simulation’, Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), vol. 12009 LNCS, no. November, pp. 12–21, 2020, doi: https://doi.org/10.1007/978-3-030-39074-7_2.
G. Gok, N. Sayar, D. Oz, H. B. Erer, A. Ekmekci, and M. Eren, ‘Comparison of 2D vena contracta area with 3D planimetric mitral valve area in rheumatoid mitral valve disease’, International Journal of Cardiovascular Imaging, vol. 36, no. 11, pp. 2115–2120, Nov. 2020, doi: https://doi.org/10.1007/s10554-019-01673-y.
Acknowledgements
The authors would like to thank Peter Paulander for the assistance during the build of the pump setup, Erik Ekbom and Muris Imsirovic at the 3D center at Skåne University Hospital in Lund for 3D-printing the phantom, and Isabella Silva Barreto for the help with tensile tests. Further, we would like to acknowledge Jonathan Berg for his help with the pressure measurement equipment, and Axel Svenningsson and Pia Sjöberg for their guidance on the software CAAS MR Solutions. Finally, Pie Medical is acknowledged for the CAAS 4D flow analysis research tool.
Funding
Open access funding provided by Lund University. The study was supported by grants from the Swedish Heart-Lung Foundation (grant no. 20220737) (PL), the Skane University Hospital's Innovation Award (PL), and by the Swedish governmental funding of clinical research (ALF) (grant no. 2022-0288) (PL). Additionally, this study was funded by the Swedish Pediatric Heart Foundation (Hjartebarnsfonden) (NH) and Skane University Hospital grant 2022/2023 (NH).
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
The conceptualization of the project was carried out by Nina Hakacova, Petru Liuba, Petter Frieberg, and Lea Christierson. The data collection was performed by Lea Christierson, Petter Frieberg, Tania Lala, Johannes Töger, and Nina Hakacova. Investigation and data analysis were performed by Lea Christierson, Petter Frieberg, Tania Lala, Nina Hakacova, Johannes Töger, Johan Revstedt, and Hanna Isaksson. Grants received by Nina Hakacova and Petru Liuba funded the study. The methodology was developed by Lea Christierson, Petter Frieberg, and Nina Hakacova, who designed the research protocols. Resources were provided by Nina Hakacova, Petter Frieberg, Johannes Töger, and Hanna Isaksson and supervision of the project was provided by Nina Hakacova, Petru Liuba, Johan Revstedt, and Hanna Isaksson. The visualization of data and drafting of the original manuscript were performed by Lea Christierson. The subsequent review, editing, and approval of the final version of the manuscript involved the contributions of all authors.
Corresponding author
Ethics declarations
Conflict of Interest
All authors certify that they are not involved in any organization or entity with any financial or non-financial interests that are directly or indirectly related to the work discussed in this manuscript.
Additional information
Communicated by Jamshid Karimov, MD, PhD.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christierson, L., Frieberg, P., Lala, T. et al. Multi-Modal in Vitro Experiments Mimicking the Flow Through a Mitral Heart Valve Phantom. Cardiovasc Eng Tech (2024). https://doi.org/10.1007/s13239-024-00732-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13239-024-00732-3