Abstract
During virus infection, viral RNAs and mRNAs function as blueprints for viral protein synthesis and possibly as pathogen-associated molecular patterns (PAMPs) in innate immunity. Here, considering recent research progress in microRNAs (miRNAs) and competitive endogenous RNAs (ceRNAs), we speculate that viral RNAs act as sponges and can sequester endogenous miRNAs within infected cells, thus cross-regulating the stability and translational efficiency of host mRNAs with shared miRNA response elements. This cross-talk and these reciprocal interactions between viral RNAs and host mRNAs are termed “competitive viral and host RNAs” (cvhRNAs). We further provide recent experimental evidence for the existence of cvhRNAs networks in hepatitis B virus (HBV), as well as Herpesvirus saimiri (HVS), lytic murine cytomegalovirus (MCMV) and human cytomegalovirus (HCMV) infections. In addition, the cvhRNA hypothesis also predicts possible cross-regulation between host and other viruses, such as hepatitis C virus (HCV), HIV, influenza virus, human papillomaviruses (HPV). Since the interaction between miRNAs and viral RNAs also inevitably leads to repression of viral RNA function, we speculate that virus may evolve either to employ cvhRNA networks or to avoid miRNA targeting for optimal fitness within the host. CvhRNA networks may therefore play a fundamental role in the regulation of viral replication, infection establishment, and viral pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, viral RNAs are regarded as either templates for translating viral proteins that exert function or as viral pathogen-associated molecular patterns (PAMPs) for detection by host pattern-recognition receptors (PRRs) in innate antiviral immunity (Goubau et al., 2013). However, several recent studies provide intriguing evidence that viral RNAs and host mRNAs with common microRNA (miRNA) binding sequences reciprocally affect each other’s levels and activities by directly competing with the targeting miRNAs. In this review, we propose a “competitive viral and host RNAs” (cvhRNAs) hypothesis, including recent studies on miRNAs and noncoding RNAs (ncRNAs) expressed by both the host and virus, as well as the logic of this new and potentially predictable virus-host interaction.
Mirnas and competitive endogenous rnas (CERNAS)
MiRNAs
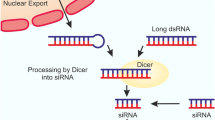
MiRNAs are a class of small noncoding double-stranded RNA molecules of approximately 22 nt in length, which regulate (usually inhibit) target gene expression at the post-transcriptional level. Full-length miRNAs (pri-miRNAs) are transcripted by RNA polymerase II, which are processed by Drosha within the nuclear compartment to produce pre-miRNAs of about 65 nucleotides in length. The pre-miRNA is then transported into the cytoplasm and further cleaved by the RNAse III-like endonuclease Dicer to produce their mature form miRNAs (Newman and Hammond, 2010). Upon loading into the RNA-induced silencing complex (RISC), which contains a member of the double-stranded RNA binding protein Argonaute family (Ago), the guide strand of the miRNA duplex recognizes and binds to conserved complementary target sites in target mRNAs (often in the 3′-UTR) through canonical base-pairing between the seed region of approximately 6- to 8- oligonucleotides. MiRNAs regulate target gene expression either by inducing their deadenylation and degradation or by leading to translational inhibition (Miyoshi et al., 2009; Hu et al., 2012). Thus far, >2500 human miRNAs (hsa-mir) and 1900 mouse miRNAs (mmu-mir) have been identified and described at the miRBase website (http://www.mirbase.org, released in Nov. 2013).
CeRNAs
Aside from small regulatory ncRNAs, such as miRNAs, the transcription machinery in mammalian cells also produces long noncoding RNAs (lncRNAs) which are typically >200–300 nt in length. Although currently the function of most of the annotated lncRNAs remains unknown, emerging studies show that certain lncRNAs are involved in various physiological processes, including cell-cycle regulation, apoptosis, and the establishment of cell identity (Ulitsky and Bartel, 2013). Functional lncRNAs may act through several mechanisms, including co-transcriptional regulation, cis- or trans-regulation of gene expression by bridging proteins and chromatin, titration of RNA-binding factors, and pairing with other RNAs to trigger post-transcriptional regulation (Ulitsky and Bartel, 2013; Augui et al., 2011). Poliseno et al. (2010) further uncovered a unique interaction of the PTEN tumor suppressor gene and its pseudogene (PTENP1), both of which are subject to the same miRNA-mediated post-transcriptional regulation (Poliseno et al., 2010). Pseudogenes harbor premature stop codons, deletions/insertions, and mutations that abrogate their translation into functional proteins. Interestingly, the PTENP1 3′UTR functions as a decoy for PTEN-targeting microRNAs due to its ability to compete for miRNA binding and thus exerts a tumor-suppressive role by modulating the de-repression of cellular levels of PTEN. A subsequent study shows that a putative PTEN competitive endogenous RNA (ceRNA), the ZEB2 transcript, which contains common miRNA recognition elements with PTEN, modulates PTEN protein levels in a protein coding-independent manner (Karreth et al., 2011). These studies indicate a new means of regulatory interaction between mRNAs (including both protein-coding and non-coding mRNAs) that have common microRNA recognition elements.
Further, more recent studies provide compelling evidence that the crosstalk between ceRNAs through competition for their shared miRNAs is involved in signaling pathways and networks in human diseases, such as autoimmune diseases, diabetes and cancer (Ling et al., 2013a, b; Fang et al., 2013; Noorbakhsh et al., 2013; Karreth and Pandolfi, 2013). Together, these newly defined mRNA-mRNA interactions have integral roles in the regulation of gene expression in various physiological and pathological processes and deepen our understanding of a novel aspect of RNA function.
The cvhrnas hypothesis
Somewhat differently from the mechanism mentioned above and unlike cellular endogenous RNAs, viral RNAs and mRNAs are exogenous in virus-infected cells. In addition, viral RNAs are mostly studied as passive targets for host miRNAs (Fig. 1A). Indeed, a large number of impressive experiments have been performed, primarily on binding site validation and functional analyses, which support the notion that the interaction between miRNAs and viral RNAs as their targets is essential to the regulation of viral RNA stability, expression, and translation, as well as viral replication and infection (Jopling et al., 2005; Shimakami et al., 2012; Song et al., 2010; Klase et al., 2012). Hosts employ their miRNAs in defense against viral infection by targeting viral products or inhibiting viral replication. Whereas viruses also encode miRNAs that target specific host genes and pathways to prolong the longevity of infected cells or evade the immune clearance, which may be beneficial for viral infectivity and/or proliferation (Carl et al., 2013; Kincaid and Sullivan, 2012).
Inference of cvhRNA cross-regulation through miRNA response elements. (A) MiRNAs guide the recognition of target mRNAs through imperfect matches with their targeting sites and thus regulate the expression of target mRNAs at the post-transcriptional level. Each miRNA has multiple (up to tens) mRNA targets. Conversely, each mRNA may harbor multiple miRNA targeting sites (Ala et al., 2013). (B) Viral RNAs and mRNAs harboring miRNA response elements exert their suppression of miRNA as sponges. Host mRNAs may act in a similar way. Thus, viral RNAs and host mRNAs could competitively sequester the same miRNA pool within infected cells. Viral RNAs as miRNA sponges can de-repress the miRNA-mediated inhibition of host mRNAs. Therefore, a cross-regulation may be formed between viral RNAs and host mRNAs that share common miRNA response elements. Stimulation (↑) or inhibition (┬) is determined following how miRNAs or RNAs impact the activities of RNAs or miRNAs
Nevertheless, viral RNAs harbor common miRNA-binding sites with host RNAs (Jopling et al., 2005; Shimakami et al., 2012; Song et al., 2010; Klase et al., 2012), indicating that viral RNAs and mRNAs potentially act as bona fide miRNA competitors with host RNAs for the same miRNA pool in infected cells. In addition, a large number of virus-encoded miRNAs and lncRNAs have been uncovered, which play a key role in persistent infections, and importantly, a significant portion of viral miRNAs mimic host miRNAs with similar target sites (Kincaid and Sullivan, 2012). Therefore, we hypothesize that viral RNAs act as sponges that can sequester endogenous miRNAs within infected cells and thus impact the stability and translational efficiency of host mRNAs with shared miRNA response elements (Fig. 1B). We term the reciprocal regulation between virus and host transcripts acting in this manner cvhRNAs.
The basis for cvhRNA crosstalk is dependent on base pairing complementarity and miRNA binding/recognition, and the ability of viral RNAs (or host mRNAs) to sequester and degrade miRNAs and the extent of de-repression in host mRNAs (or viral RNAs) by miRNA down-regulation. Down-regulation of miRNA by interacting viral or host mRNAs could be either via sequestration/sponge or destabilization/degradation, or both (Torres et al., 2011), although the exact mechanisms await further investigation. Thus, the relative abundance of virus vs. host RNAs, levels of common miRNAs, and the number of miRNA response elements may all contribute to cvhRNA interactions, according to a mathematical mass-action model for ceRNA networks (Ala et al., 2013). In addition, the intracellular localization of virus/host mRNAs and miRNAs, the extent of base pairing complementarity of the competitive RNAs, as well as the time-course of viral RNA expression may also impact ceRNA networks. Given that viruses usually generate highly redundant transcripts upon infection and that multiple highly abundant miRNAs interact with viral RNAs or mRNAs (Shimakami et al., 2012; Kincaid and Sullivan, 2012), we expect that reciprocal interactions between viral RNA-miRNA-host mRNA may orchestrate a permissive molecular environment that enables the generation of cvhRNA networks under virus infection.
The experimental evidence for cvhrna networks
The existence of cvhRNA cross-talk in viral infections
An early indication that virus-encoded RNAs are involved in regulating host miRNA abundance came from Cazalla et al. (2010), who demonstrated that Herpesvirus saimiri (HVS)-expressed noncoding RNAs have certain miRNA binding elements and can interact with host miRNAs in virally transformed T cells. They found that viral ncRNAs down-regulate mature miR-27 in a binding-dependent manner. Transient knockdown of one of the ncRNAs, HSUR 1, resulted in a decreased level of the miR-27 target protein, FOXO1 (Cazalla et al., 2010). Subsequently, several impressive experiments have been performed on the role of coding RNAs and ncRNAs on the degradation and decay of host miRNAs. Lytic murine cytomegalovirus (MCMV) infection leads to rapid degradation of cellular miR-27a and miR-27b via expression of a spliced and highly abundant protein-coding transcript, m169, which facilitates efficient virus replication (Marcinowski et al., 2012). Interestingly, MCMV infection up-regulates expression of genes in mitogen-activated protein kinases (MAPK) pathway, specifically p38, which enhances viral replication (Tang-Feldman et al., 2013). Up-regulation of p38 may attribute to decreased miR-27a as p38 is a potential target of miR-27a. Similarly, a viral intergenic ncRNA element of human cytomegalovirus (HCMV) mediates the selective down-regulation of mature miR-17 and miR-20a through sequence-specific interactions. Viral RNA-induced miR-17 decay is essential for rapid viral production, indicating a potential role for the interaction between the viral ncRNA and cellular miRNAs during viral pathogenesis (Lee et al., 2013a). Indeed, IL-8 as a target of miR-17/20a (Yu et al., 2010), significantly stimulates HCMV replication through cytokine-stimulated transcription of viral late genes and activation of early events in viral replication (Szabó et al., 1999; Redman et al., 2002).
Hepatitis B virus (HBV), a major hepatotropic DNA virus, chronically infects ~350 million people worldwide. Cellular miRNAs have a striking role in mediating HBV replication and infection (Hu et al., 2012; Zhang et al.,2013). MiR-122 is the most abundant liver-specific miRNA, which reaches approximately 70% of the total miRNA population in the adult liver. We observed a significant decrease of miR-122 levels under HBV infection. We further demonstrated that a miR-122 binding element exists in the 3′-UTR of all four HBV mRNAs (i.e., the pre-C/C or pre-genomic RNA (pgRNA), pre-S, S, and X mRNAs), and these viral RNAs act as sponges to bind and sequester endogenous miR-122, which led to decreased miR-122. Furthermore, expression of the HBV 3′-UTR-containing luciferase reporter was sufficient to up-regulate expression of two cellular genes, cyclin G1 and pituitary tumor-transforming gene 1 (PTTG1) binding factor (PBF), whose 3′-UTRs contain a common miR-122 response element as HBV RNAs (Li et al., 2013; Wang et al., 2012). A follow-up study revealed that cyclin G1 significantly enhances HBV expression and replication by suppressing p53-mediated inhibition of HBV enhancers, indicating that the cross-regulation of HBV RNAs and cyclin G1 expression possibly contribute to viral persistence. Further, up-regulation of PBF may contribute to HBV infection-related hepatocellular carcinoma (HCC) because PBF interacts with PTTG1 and increases its transcriptional activity at multiple oncogenes (Li et al., 2013), as well as promotes cell proliferation, invasion, and HCC tumor growth in mice. Under HBV infection, the HBV RNA copy number per cell reaches up to 105 (Li et al., 2013; Wang et al., 2012), and miR-122 is expressed in hepatocytes at 50,000 copies per cell (Hu et al., 2012; Filipowicz and Grosshans, 2011). Conceivably, a positive cross-regulation between HBV RNAs and cyclin G1 or PBF occurs in the presence of the large amounts of viral RNAs which efficiently sequester miR-122, resulting in intermediate miR-122 levels in hepatocytes.
Interestingly, we also identified an IFN-stimulated gene (ISG), NT5C3, which efficiently sequesters miR-122 with its mRNA 3′-UTR through binding sequence-specific interactions (Hao et al., 2013). Conceivably, the possible HBV RNAs-NT5C3 mRNA crosstalk may be a counter mechanism that HBV evolved to antagonize the antiviral activity of IFN.
In addition to the interaction between HBV RNAs and host mRNAs that share a common miR-122 response element, two other groups more recently reported another example of cvhRNA crosstalk in HBV infection (Liu et al., 2013; Wang et al., 2013b). They found that the HBx transcript and HBV mRNAs directly trigger the down-regulation of miR-15a/16 via the miRNA targeting sequences in the viral RNA, and as a consequence, the target of miR-15a/16 (Bcl-2) is up-regulated. Up-regulated Bcl-2 then inhibits the downstream cascade of apoptosis in hepatoma cells. These studies identify a novel HBx transcript and the HBV mRNAs-miR-15a/16-Bcl-2 regulatory pathway that is involved in apoptosis inhibition, which may help facilitate HBV infection and HCC development.
Together, the above studies provide experimental evidence for the existence of cvhRNA networks during HBV infection (Fig. 2). CvhRNAs, including HBV RNAs (e.g., pgRNA, pre-S, S, and X mRNAs) and host mRNAs (e.g., cyclin G1, PBF, NT5C3, and Bcl-2), harbor shared response elements for certain abundant cellular miRNAs (e.g., miR-122 and miR-15a/miR-16). When viral RNAs are highly produced during HBV infection, the repression of host target genes conferred by these common miRNAs can be diluted as viral RNAs down-regulate common miRNAs. Up-regulation of host target genes would contribute to enhanced HBV expression and replication, persistent viral infection, and HCC development.
Schematic figure of how cvhRNA networks may mediate viral replication, infection, and the development of HCC during HBV infection. In hepatocytes, miR-122 effectively suppresses the expression of its target genes (cyclin G1, PBF, and NT5C3) at the post-transcriptional level by binding to the miRNA response element within the 3′-UTR of target mRNAs. During HBV infection, high levels of viral RNAs harboring a miR-122 response element in their 3′-UTR competitively sponge and efficiently sequester cellular miR-122, thus blocking the binding of miR-122 to its host target mRNAs. In this manner, viral RNAs de-repress and rescue the expression of host target mRNAs. Similarly, HBV RNAs positively cross-regulate Bcl-2 expression through shared miR-15a/16 response elements. The dose effect between competitive HBV RNAs and host mRNAs is shown. In the networks consisting of four viral RNAs (pgRNA, pre-S, S, and X mRNAs) and four host mRNAs (cyclin G1, PBF, NT5C3, and Bcl-2), elevated expression of de-repressed host genes contributes to enhanced HBV replication, persistent viral infection, and HCC development
HCV (hepatitis C virus), another main hepatotropic positive-sense RNA virus, causes chronic infection in about 180 million people worldwide. Extensive studies have demonstrated that miR-122 acts in an unusual manner to stimulate the replication and expression of HCV by binding to two closely spaced target sites in the 5′-UTR of the HCV genome (Jopling et al., 2005; Shimakami et al., 2012; Pang et al., 2012). In this context, interaction of miR-122 and viral RNA stabilizes viral RNA and decreases its decay, rather than induces its degradation as most miRNAs do. Notably, Shan et al. (2007) showed that inhibition of miR-122 up-regulates Heme oxygenase-1 (HO-1) which significantly inhibits HCV replication, suggesting that miR-122 promotes HCV replication partly via down-regulation of HO-1 (Shan et al., 2007). Considering that HCV RNA also interacts with miR-122, it is possible that the positive regulatory pathway of HCV RNA-miR-122-HO-1 may contribute to enhanced HCV replication.
In HBV and HCV infections, robust viral specific T cell responses are observed during resolution of acute and self-limited HBV infections with viral clearance, whereas chronic infection with viral persistence is characterized by only weak and impaired T-cell response. T cells are therefore believed to play a critical role in the control of HBV and HCV replication and infection (Manigold and Racanelli, 2007; Wang et al., 2013a). Conceivably, cvhRNA networks may play an important role in regulation of host immune responses in the liver microenvironment that induce immune tolerance towards viruses. Indeed, the above preliminary studies have shown that the cross-talk and reciprocal interactions between HBV/HCV RNAs and host mRNAs contribute to enhanced and persistent viral expression and replication which inevitably drive T cell tolerance and exhaustion by long-term intensive viral antigenic stimulation. Moreover, it is possible that cvhRNA networks in HBV or HCV infection may directly or indirectly affect expressions of co-inhibitory molecule programmed death (PD)-1 on T cells and its ligand PD-L1 on dendritic cells (DCs), the cytotoxic T lymphocyte antigen-4 (CTLA-4) and proapoptotic protein Bcl2-interacting mediator (Bim) on T cells, or activities of Tregs in the liver microenvironment. Further understanding the regulation of cvhRNA networks in influencing the outcome of viral infections (e.g. viral clearance or persistent infection) will allow the development of novel strategies for therapeutic regimen by targeted reversal of tolerising mechanisms.
Prediction of potential cross-regulation between host and virus by cvhRNA hypothesis
In principle, any viral RNAs sharing common miRNA response elements with host mRNAs could potentially act as cvhRNAs and form regulatory networks across the transcriptome. Besides HSV, MCMV and HCMV, we speculate that other examples of cvhRNA may also exist in viral infections (Table. 1). For instance, HIV-1 may probably up-regulate Notch-3 through cvhRNA crosstalk, as both HIV-1 gag-pol and Notch-3 mRNAs share a common miR-150 response sequence (Huang et al., 2007; Ghisi et al., 2011), and HIV-1 infection down-regulates miR-150 (Swaminathan et al., 2009). Notch activation in turn is involved in HIV-associated nephropathy (Sharma et al., 2010). The cvhRNA hypothesis also predicts another possible reciprocal interaction between HIV nef and host PTEN mRNAs that have a shared miR-29a response sequence. HIV infection may induce apoptosis of CD4+ T lymphocytes through upregulating PTEN (Kong et al., 2011; Sun et al., 2012; Dabrowska et al., 2008). Similarly, influenza virus WSN infection induces up-regulation of MMP9 which mRNA shares a common miR-491-5p binding site with viral PB1 mRNA (Song et al., 2010; Lee et al., 2013b; Yuan et al., 2013), indicating the potential cross-regulation between viral mRNA and host mRNA. Furthermore, the notion of cvhRNAs was recently linked to human papillomaviruses (HPVs) (Gunasekharan and Laimins, 2013). HPV-31, whose E1 and E2 open reading frames contain miR-145 binding sequences, could lead to a dramatic decrease miR-145 level and increased the expression of a miR-145 target, the cellular transcription factor KLF-4. HPV-regulated KLF-4 may contribute to the control of viral life cycle during differentiation.
As cvhRNA networks primarily depend on viral mRNA abundance, different mechanisms responsible for mRNA production among different virus families above are listed in Table 2. Among these virus types, ssRNA, dsRNA or dsDNA genomes serve as the template for mRNA transcription, some of which even contain a reverse transcription stage in their replication cycle. This indicates that cvhRNA networks may exist universally among viruses with different types of genomes (e.g. ssRNA, dsRNA and dsDNA).
Conclusions and outlook
In conclusion, we speculate that cross-talk between viral RNAs and host mRNAs via common miRNA response elements forms complex networks during virus infection, which may affect viral replication, persistence, and pathogenesis. This expands the knowledge of how viral RNAs and mRNAs function in multiple ways, not merely as blueprints for viral protein synthesis or as PAMPs in innate immunity. Figs. 1 and 2 only show the direct interactions between viral RNAs and host mRNAs. In general, one miRNA has several or even multiple RNA/mRNA targets, and conversely, one target RNA or mRNA likely harbors several miRNA response elements. Thus, indirect interactions between viral RNAs and host mRNAs may also play an important role in cvhRNA networks, which may be further intimately intertwined by cellular transcription factor networks (Ala et al., 2013). In addition, variations in miRNA response elements (e.g. the seed region of pairing) of viral RNA which inevitably affect base pair complementarity, could thereby influence the interactions between viral and host RNAs. To establish infection, viruses likely evolved to accumulate mutation to increase the number of shared miRNA response elements with supportive host factors and decrease the number shared with inhibitory host factors; this hypothesis deserves further investigation. Global mRNA and miRNA sequencing, Gene Ontology analysis of transcriptomic databases and establishment of proper mathematical models are needed to provide further dissection of the impact of cvhRNA networks on virus and host interaction.
A fine-tuned balance between miRNAs and host mRNA targets (including coding and noncoding transcripts) likely exists in cells with no viral infection (Ala et al., 2013). Conceivably, viral infection disturbs mRNA homeostasis through cvhRNA networks, as shown in Fig. 3. Information regarding the balance between miRNA pools and host mRNA homeostasis under viral infection is essential to understand the molecular basis for viral persistence and pathogenesis, which may facilitate the development of new antiviral strategies.
Potential alteration of host mRNA homeostasis by viral RNAs. Many mammalian viruses produce high levels of redundant viral RNAs and mRNAs. According to the cvhRNAs hypothesis, under certain conditions for cvhRNA activity in viral infections, these viral RNAs could modify the balance between cellular miRNAs and host mRNA targets in a miRNA response sequence dependent and coding independent manner. Viral RNAs directly result in the depression of cellular transcripts with shared miRNA response elements and the subsequent aberrant expression of host proteins
An intriguing question arises from the hypothesis of cvhRNA regulatory networks is why virus evolves to facilitate rather than avoid miRNA binding which inevitably leads to repression of viral RNA function. We speculate that virus may employ either strategy (to usurp cvhRNA networks or to avoid miRNA targeting) for the establishment of persistent infection, depending on which strategy plays a major role in favoring viral infectivity and replication. In particular, some viruses produce redundant RNA/mRNAs during infections as seen in HBV infection (Li et al., 2013; Wang et al., 2012), which facilitate to form cvhRNA networks.
As emerging studies reveal that cvhRNAs play essential roles in viral infection and pathogenesis, it will be important to take into consideration the unique features of direct communication between viral RNAs and host mRNAs when studying the interaction between the virus and host in the long run. We hope that this review of recent advances on ceRNA and cvhRNA networks will serve to stimulate more interest and experimental activity and re-define the concept of virus-host interactions as more than protein-mediated regulation.
Abbreviations
- ceRNAs:
-
competitive endogenous RNAs
- cvhRNAs:
-
competitive viral and host RNAs
- PAMPs:
-
pathogen-associated molecular patterns
- ncRNAs:
-
noncoding RNAs
- HVS:
-
Herpesvirus saimiri
- MCMV:
-
cytomegalovirus
- HCMV:
-
human cytomegalovirus
- HPVs:
-
human papillomaviruses
References
Ala U, Karreth FA, Bosia C, Pagnani A, Taulli R, Léopold V, Tay Y, Provero P, Zecchina R, Pandolfi PP (2013) Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments. Proc Natl Acad Sci USA 110:7154–7159
Augui S, Nora EP, Heard E (2011) Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet 12:429–442
Carl JW Jr, Trgovcich J, Hannenhalli S (2013) Widespread evidence of viral miRNAs targeting host pathways. BMC Bioinf 14(Suppl 2):S3
Cazalla D, Yario T, Steitz JA (2010) Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328:1563–1566
Dabrowska A, Kim N, Aldovini A (2008) Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes. J Immunol 181:8460–8477
Fang L, Du WW, Yang X, Chen K, Ghanekar A, Levy G, Yang W, Yee AJ, Lu WY, Xuan JW et al (2013) Versican 3′-untranslated region (3′-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. FASEB J 27:907–919
Filipowicz W, Grosshans H (2011) The liver-specific microRNA miR-122: biology and therapeutic potential. Prog Drug Res 67:221–238
Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, Mussolin L, Ruggero K, Bonanno L, Guffanti A et al (2011) Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood 117:7053–7062
Goubau D, Deddouche S, Reis E, Sousa C (2013) Cytosolic sensing of viruses. Immunity 38:855–869
Gunasekharan V, Laimins LA (2013) Human papillomaviruses modulate microRNA 145 expression to directly control genome amplification. J Virol 87:6037–6043
Hao J, Jin W, Li X, Wang S, Zhang X, Fan H, Li C, Chen L, Gao B, Liu G et al (2013) Inhibition of alpha interferon (IFN-α)-induced microRNA-122 negatively affects the anti-hepatitis B virus efficiency of IFN-α. J Virol 87:137–147
Hu J, Xu Y, Hao J, Wang S, Li C, Meng S (2012) MiR-122 in hepatic function and liver diseases. Protein Cell 3:364–371
Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H et al (2007) Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 13:1241–1247
Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309:1577–1581
Karreth FA, Pandolfi PP (2013) ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov 3:1113–1121
Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA et al (2011) In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147:382–395
Kincaid RP, Sullivan CS (2012) Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog 8:e1003018
Klase Z, Houzet L, Jeang KT (2012) MicroRNAs and HIV-1: complex interactions. J Biol Chem 287:40884–40890
Kong G, Zhang J, Zhang S, Shan C, Ye L, Zhang X (2011) Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS ONE 6:e19518
Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, Kim D, Baek D, Ahn K (2013a) Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell Host Microbe 13:678–690
Lee YH, Lai CL, Hsieh SH, Shieh CC, Huang LM, Wu-Hsieh BA (2013b) Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res 178:411–422
Li C, Wang Y, Wang S, Wu B, Hao J, Fan H, Ju Y, Ding Y, Chen L, Chu X et al (2013) HBV mRNAs-mediated miR-122 inhibition up-regulates PTTG1-binding protein which promotes HCC tumor growth and cell invasion. J Virol 87:2193–2205
Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Li Y, Li Y, Ye Y (2013a) Dickkopf-1 (DKK1) phosphatase and tensin homolog on chromosome 10 (PTEN) crosstalk via microRNA interference in the diabetic heart. Basic Res Cardiol 108:352
Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Ye Y (2013b) MicroRNA-dependent cross-talk between VEGF and HIF1α in the diabetic retina. Cell Signal 25:2840–2847
Liu N, Zhang J, Jiao T, Li Z, Peng J, Cuizhu Q, Ye X (2013) Hepatitis B virus inhibits apoptosis of hepatoma cells by sponging miR-15a/16 cluster. J Virol 87:13370–13378
Manigold T, Racanelli V (2007) T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect Dis 7:804–813
Marcinowski L, Tanguy M, Krmpotic A, Rädle B, Lisnić VJ, Tuddenham L, Chane-Woon-Ming B, Ruzsics Z, Erhard F, Benkartek C et al (2012) Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog 8:e1002510
Miyoshi K, Okada TN, Siomi H, Siomi MC (2009) Characterization of the miRNA-RISC loading complex and miRNA-RISC formed in the Drosophila miRNA pathway. RNA 15:1282–1291
Newman MA, Hammond SM (2010) Emerging paradigms of regulated microRNA processing. Genes Dev 24:1086–1092
Noorbakhsh J, Lang AH, Mehta P (2013) Intrinsic noise of microRNA-regulated genes andthe ceRNA hypothesis. PLoS ONE 8:e72676
Pang PS, Pham EA, Elazar M, Patel SG, Eckart MR, Glenn JS (2012) Structural map of a Pan PS, microRNA-122: hepatitis C virus complex. J Virol 86:1250–1254
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Redman TK, Brittm WJ, Wilcox CM, Graham MF, Smith PD (2002) Human cytomegalovirus enhances chemokine production by lipopolysaccharide-stimulated lamina propria macrophages. J Infect Dis 185:584–590
Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL (2007) Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology 133:1166–1174
Sharma M, Callen S, Zhang D, Singhal PC, Vanden Heuvel GB, Buch S (2010) Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS 24:2161–2170
Shimakami T, Yamane D, Jangra RK, Kempf BJ, Spaniel C, Barton DJ, Lemon SM (2012) Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Natl Acad Sci USA 109:941–946
Song L, Liu H, Gao S, Jiang W, Huang W (2010) Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J Virol 84:8849–8860
Sun G, Li H, Wu X, Covarrubias M, Scherer L, Meinking K, Luk B, Chomchan P, Alluin J, Gombart AF et al (2012) Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res 40:2181–2196
Swaminathan S, Zaunders J, Wilkinson J, Suzuki K, Kelleher AD (2009) Does the presence of anti-HIV miRNAs in monocytes explain their resistance to HIV-1 infection? Blood 113:5029–5030
Szabó J, Bácsi A, Beck Z, Kiss J, Andirkó I, Tóth FD (1999) Role of interleukin-8 and transforming growth factor-beta1 in enhancement of human cytomegalovirus replication by human T cell leukemia-lymphoma virus type I in macrophages co-infected with both viruses. J Interferon Cytokine Res 19:209–217
Tang-Feldman YJ, Lochhead SR, Lochhead GR, Yu C, George M, Villablanca AC, Pomeroy C (2013) Murine cytomegalovirus (MCMV) infection upregulates P38 MAP kinase in aortas of Apo E KO mice: a molecular mechanism for MCMV-induced acceleration of atherosclerosis. J Cardiovasc Transl Res 6:54–64
Torres AG, Fabani MM, Vigorito E, Gait MJ (2011) MicroRNA fate upon targeting with anti-miRNA oligonucleotides as revealed by an improved Northern-blot-based method for miRNA detection. RNA 17:933–943
Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154:26–46
Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen L, Wu E, Ye X, Gao GF, Wang F et al (2012) Loss of MiR-122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G1 modulated P53 activity. Hepatology 55:730–741
Wang S, Zhang X, Ju Y, Zhao B, Yan X, Hu J, Shi L, Yang L, Ma Z, Chen L et al (2013a) MicroRNA-146a feedback suppresses T cell immune function by targeting Stat1 in patients with chronic hepatitis B. J Immunol 191:293–300
Wang Y, Jiang L, Ji X, Yang B, Zhang Y, Fu XD (2013b) Hepatitis B viral RNA directly mediates down-regulation of the tumor suppressor microRNA miR-15a/miR-16-1 in hepatocytes. J Biol Chem 288:18484–18493
Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, McCue PA, Quong AA, Lisanti MP, Pestell RG (2010) microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci USA 107:8231–8236
Yuan M, Zhan Q, Duan X, Song B, Zeng S, Chen X, Yang Q, Xia J (2013) A functional polymorphism at miR-491-5p binding site in the 3′-UTR of MMP-9 gene confers increased risk for atherosclerotic cerebral infarction in a Chinese population. Atherosclerosis 226:447–452
Zhang X, Hou J, Lu M (2013) Regulation of hepatitis B virus replication by epigenetic mechanisms and microRNAs. Front Genet 4:202
Acknowledgements
This work was supported by a grant from the National Basic Research program (973 Program) (No. 2014CB542602) and grants from the National Natural Science Foundation of China (Grant Nos. 31230026, 81321063, 81102018).
Compliance with ethics guidelines
Changfei Li, Jun Hu, Junli Hao, Bao Zhao, Bo Wu, Lu Sun, Shanxin Peng, George F. Gao and Songdong Meng declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Li, C., Hu, J., Hao, J. et al. Competitive virus and host RNAs: the interplay of a hidden virus and host interaction. Protein Cell 5, 348–356 (2014). https://doi.org/10.1007/s13238-014-0039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13238-014-0039-y