Abstract
Epigallocatechin gallate (EGCG), the primary catechin in green tea, has improved cholesterol metabolism. However, the molecular mechanisms of EGCG underlying these functions are not fully understood. In this study, we aimed to investigate the molecular mechanisms underlying EGCG’s effect on low-density lipoprotein (LDL) in HepG2 cells. Real-time PCR and Western blot analysis were used to determine the mRNA and protein levels in the human hepatoma cell line (HepG2). LDL uptake assay was used to quantify the low-density lipoprotein receptor (LDLR) function. EGCG induced significantly up-regulated LDLR protein and mRNA levels in HepG2 cells (P < 0.05). Both at the transcriptional level and at the protein level, EGCG can significantly (P < 0.05) down-regulate the elevated expression levels of liver X receptor α (LXRα) and inducible degrader of the LDLR (Idol) due to 25-OHC. Fluorescence results showed that EGCG induction could also significantly increase LDL uptake (P < 0.05). EGCG regulates LDL uptake through the LXRα-LDLR pathway, and EGCG can effectively improve the abnormal expression of protein and mRNA induced by 25-OHC.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of human death. Excessive low-density lipoprotein cholesterol (LDL-c) is the most significant risk factor for the development of atherosclerosis (AS) and ASCVD [11, 16]. The low-density lipoprotein receptor (LDLR) is a cell surface receptor expressed primarily in the liver that clears LDL particles from plasma through receptor-mediated endocytosis. Inadequate LDLR function can lead to an increase in plasma LDL-c concentration and premature atherosclerosis [6]. Although statins can reduce cardiovascular events by lowering LDL-c levels, they are highly variable and reduce patient adherence to treatment, so additional or alternative LDL-c-lowering treatments are urgently needed [18]. The discovery of the protein invertase subtilin/ketoin type 9 (PCSK9) at the beginning of this century effectively made up for the lack of statins, PCSK9 is a plasma protein mainly produced and secreted by the liver, which can affect the plasma concentration of LDL-c by regulating LDLR, PCSK9 inhibitors are important new drugs to reduce LDL-c levels and treat ASCVD [17]. PCSK9 is the main post-translational regulator of LDLR, in addition, myosin regulates light chain interacting protein (Mylip)/inducible degrader of the LDLR (Idol) (also known as Mylip/Idol) is also LDL translation regulator, PCSK9 and Mylip/Idol proteins enhance LDLR degradation, thereby hindering LDL clearance from plasma [12]. PCSK9 and Mylip/Idol proteins induced LDLR degradation, and it is of great significance to elucidate the effect of LDLR on disease progression.
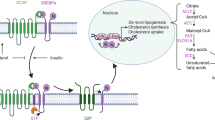
PCSK9 binds to the extracellular domain of LDLR on the surface of hepatocytes and interferes with LDLR recycling back into the cell membrane after endocytosis [5]. Blood PCSK9 levels were positively correlated with AS risk, and PCSK9 relies on lipid regulation and inflammatory regulation to promote the development of AS. The increase in LDL-c concentration in the blood is the main reason for the increase of lipids, LDLR can bind to LDL-c to reduce the level of LDL-c in the blood, and the liver abnormally produces a large amount of PCSK9, and the increased PCSK9 binds to the LDL-c-LDLR dimer and promotes its degradation in lysosomes through the nest protein-dependent mechanism, resulting in downregulation of LDLR on the surface of hepatocytes, which will aggravate the increase of blood LDL-c concentration [1]. The inflammatory modulation effect of PCSK9 is mainly reflected in promoting the differentiation of T cells into Th1 and Th17 subsets, resulting in increased secretion of inflammatory factors such as interferon γ (IFN-γ) and interleukin-17A (IL-17A), while promoting the interaction between LDLR and T cell receptor (TCR), and promoting the differentiation of CD8 + T cells into cytotoxic T lymphocytes [15]. As another important degradant of LDLR, Idol is an E3 ubiquitin ligase, which can trigger ubiquitination of the LDLR cytoplasmic domain, promote lysosomal degradation and trigger LDLR degradation [7]. Idol is regulated by another sterol-dependent nuclear receptor, liver X receptor α (LXRα), which is activated when cellular cholesterol is excessive, and the mechanism of Idol regulating LDLR is shown in Fig. 1 [24]. At present, most of the relevant research on LDLR focuses on PCSK9, while the relevant research on LDLR regulation by Idol is not sufficient, and the relevant mechanism of Idol regulation of LDLR still needs to be further elucidated.
Hepatocytes Idol regulates LDL-c related mechanisms. After the activation of LXR in the nucleus of hepatocytes, it promotes the increase of Idol expression. Extracellular LDL-c and cell membrane indicate LDLR binding, LDLR carries LDL-c to hepatocyte lysosomes for breakdown, and then LDLR continues back to the cell membrane surface to bind to new LDL-c. LDLR binds to Idol during its return to the surface of the cell membrane, resulting in a decrease in LDLR quantity and lower LDL-c uptake
Green tea, or unfermented tea, is a beverage consumed worldwide with the highest content of flavonoids. Studies have shown that regular consumption of green tea can reduce cardiovascular disease (CVD) morbidity and mortality [19, 22]. Catechins are the main forms of flavonoids and are composed of epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) [2]. Due to its potency and relative content, EGCG is considered the main active compound in green tea [9]. Study has shown that EGCG can affect LDLR and PCSK9 expression [23], and whether EGCG can affect Idol expression is not clear, and the detailed molecular mechanism of EGCG activating LDLR is not clear.
Hence, the aim of this study was to elucidate the effect of EGCG on LDLR and Idol expression in HepG2 cells, thus providing a theoretical basis for the development of EGCG as a new drug for the treatment of ASCVD.
Materials and methods
Materials
Antibodies specific to the following proteins were obtained from Abcam (UK): LDLR (Abcam, #ab52818), LXRα (Abcam, #ab176323) and Idol (Abcam, #ab74562). GAPDH (#2118S, 1:1000) was obtained from Cell Signaling Technology (USA). EGCG (Sigma, #E4143) was obtained from Sigma (USA). Dil-LDL (Yiyuan biotechnology, # YB-0011) was purchased from Yiyuan biotechnology (China).
Cell culture
The human hepatoma cell line HepG2 was obtained from American Type Culture Collection and cultured in minimum essential eagle medium supplemented with 10% fetal bovine serum (FBS). All cells were maintained in a 5% CO2 humidified atmosphere at 37 ℃.
RNA preparation and real-time qPCR
The RNA was isolated with Trizol (Invitrogen Life Technologies, California, USA) and reverse-transcribed to cDNA. The mRNA expression of LDLR, LXRα and Idol was determined by real-time PCR using specific primers, which were obtained from NCBI website (https://www.ncbi.nlm.nih.gov/). The SYBR Green real-time quantitative PCR assays were performed on a Lightcycler 480 II instrument (Roche Applied Science), and the melt curve was done. The quantitative analysis of mRNA expression was performed via 2−ΔΔCt method. The primer sequences are listed in Table 1.
Western blotting
At each time point, cells were harvested, washed with PBS, and homogenized with lysis buffer (50 mM Tris–HCl, pH 8.0, 5 mM EDTA, 150 mM NaCl, 0.5% NP-40, 0.5 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride), followed by shaking for 30 min at 4 ℃. Lysates were then centrifuged at 12,000 g for 10 min and supernatant was collected and quantified. Equal amounts of protein extract (20 μg or as indicated) were electrophoresed on a sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to a PVDF membrane (Millipore). The membrane was blocked with Tris-buffered Solution (20 mM Tris–HCl, 150 mM NaCl, and 0.1% Tween-20) containing 5% BSA for 1 h and probed with antibodies specific for LDLR (1:3000), LXRα (1:1000), Idol (1:1000) and GAPDH (1:10,000), respectively, at 4℃ overnight, followed by incubation with the corresponding secondary antibodies (Proteintech, Chicago, USA). The bands were visualized with enhanced chemiluminescence (ECL) on a Fuji LAS4000 (GE Healthcare).
LDL uptake assay
HepG2 cells were incubated in serum-deficient medium for 24 h, then 25-OHC was added to the serum-containing medium for an additional 30 min, followed by the addition of EGCG of different concentrations (10, 25, 50 μM).To measure LDL uptake, Dil-LDL (20 μg/mL) (with red fluorescence) was added, followed by incubation at 37℃ for 4.5 h. The cells were washed with phosphate-buffered saline (PBS) three times. The LDLR activity of the HepG2 cells was determined by detecting the red fluorescence of the cells via a fluorescence microscope. The average fluorescence intensity was calculated by ImageJ software for quantitative analysis of LDL-uptake.
Statistical analysis
The continuous variables were presented as mean ± SEM. Student’s t-test was used to compare the difference between the two groups, and one-way ANOVA followed by Dunnett’s Multiple Comparison Test was used to compare the difference for more than two groups. P < 0.05 was considered statistically significant.
Results
EGCG increased the amount of LDLR protein and mRNA in HepG2 cells
25-OHC inhibits the expression of LDLR proteins and mRNA. We used Western blot to investigate whether EGCG affects LDLR protein levels, and further verified whether EGCG can restore LDLR protein expression while 25-OHC inhibits LDLR protein expression (Fig. 2A, B). Real-time PCR detected the abundance of LDLR mRNA in HepG2 cells exposed to EGCG for 24 h and verified whether EGCG played a role in restoring LDLR mRNA expression (Fig. 2C, D). We observed concentration-dependent upregulation of LDLR protein levels in EGCG-treated cells alone (Fig. 2A), and 25-OHC (10 μmol/L) treatment reduced LDLR protein expression by 50% (P < 0.05) compared to DMEM-cultured HepG2 cells, and co-treatment with 25-OHC and EGCG significantly (P < 0.05) upregulated LDLR protein levels (Fig. 2B). LDLR mRNA levels in EGCG-treated cells also showed a significant upward trend with the increase of EGCG concentration (P < 0.05) (Fig. 2C), 25-OHC reduced LDLR mRNA expression by 51%, and 25-OHC and EGCG co-treatment also significantly upregulated LDLR mRNA levels (P < 0.05) (Fig. 2D).
EGCG promotes LDLR expression and 25-OHC inhibits this effect. A, B WB assays shows that the expression of LDLR protein in HepG2 cells can be promoted by EGCG treatment, and this effect can be reversed by 25-OHC. C, D RT-qPCR shows that EGCG facilitates mRNA level of LDLR, and 25-OHC supplement can abate EGCG-induced increased LDLR mRNA level. *P < 0.05
EGCG exerted a down-regulation effect on the expression of Idol
Idol expression is detected at both mRNA and protein levels. In HepG2 cells cultured at different concentrations of EGCG only, there was no significant difference in the expression of Idol protein (P > 0.05) (Fig. 3A). Compared to DMEM-cultured HepG2 cells, 25-OHC (10 μmol/L) increased the expression of Idol protein (P < 0.05). The combination of 25-OHC and EGCG significantly downregulated Idol protein in a dose-dependent manner (P < 0.05) (Fig. 3B). There was also no significant difference in Idol mRNA when treated with EGCG alone (P > 0.05) (Fig. 3C). Idol mRNA expression was increased by 80% after 25-OHC culture compared to DMEM-cultured HepG2 cells (P < 0.05). The combination of 25-OHC and EGCG significantly downregulated Idol mRNA compared to 25-OHC treatment alone and was dose-dependent (P < 0.05) (Fig. 3D).
Idol expression in HepG2 cells cannot be affected by EGCG, but can be down-regulated by the combination of EGCG with 25-OHC. A, B WB assays shows that the expression level of Idol protein in HepG2 cells cannot be affected by EGCG treatment, but can be significantly down-regulated by 25-OHC combined with EGCG. C, D RT-qPCR shows that EGCG have no impact on mRNA level of Idol, but 25-OHC combined with EGCG can decrease Idol mRNA level. *P < 0.05
EGCG exerted a down-regulation effect on the expression of LXRα
Since EGCG downregulated the expression of Idol, we detected changes in the upstream nuclear receptor LXRα of Idol. Only in HepG2 cells cultured at different concentrations of EGCG, there was no significant difference in the expression of LXRα protein (P > 0.05) (Fig. 4A). Compared with DMEM-cultured HepG2 cells, 25-OHC (10 μmol/L) treatment increased LXRα protein expression by 67% (P < 0.05), and 25-OHC and EGCG co-treatment significantly downregulated LXRα protein levels (P < 0.05) (Fig. 4B). In EGCG-cultured HepG2 cells, there was no significant difference in expression levels at LXRα mRNA (P > 0.05) (Fig. 4C). The expression of LXRα mRNA was increased by 25-OHC treatment, and it was significantly downregulated after co-treatment with 25-OHC and EGCG (P < 0.05) (Fig. 4D).
LXRα expression in HepG2 cells cannot be affected by EGCG, but can be down-regulated by the combination of EGCG with 25-OHC. A, B WB assays shows that the expression level of LXRα protein in HepG2 cells cannot be affected by EGCG treatment, but can be significantly down-regulated by 25-OHC combined with EGCG. C, D RT-qPCR shows that EGCG have no impact on mRNA level of LXRα, but 25-OHC combined with EGCG can decrease LXRα mRNA level. *P < 0.05
EGCG-induced LDL-uptake in HepG2 cells
Dil-LDL (with red fluorescence) uptake experiments were used to investigate the effect of EGCG on the uptake of extracellular LDL by HepG2 cells. Treatment with EGCG alone, we found that EGCG induced LDL uptake in a concentration-dependent manner (P < 0.05) (Fig. 5A). 25-OHC inhibited LDL uptake, and co-treatment with EGCG weakened the inhibition of LDL uptake, and EGCG treatment significantly increased LDL absorption, which was associated with LDLR protein changes (P < 0.05) (Fig. 5B).
EGCG induces LDL-uptake in HepG2 cells, which can be reversed by 25-OHC. A LDL uptake assay shows that the ability of LDL-uptake in HepG2 cells can be promoted by EGCG. B 25-OHC supplement can inhibit the promotion of LDL-uptake in HepG2 cells induced by EGCG. White arrow represents EGCG uptake. *P < 0.05 (color figure online)
Discussion
The results of this study showed that EGCG, the main component in green tea, has the effect of regulating the expression of LXRα, Idol and LDLR in HepG2 cells. The effects of EGCG on the expression of LXRα, Idol and LDLR can be reflected at the transcriptional level and protein level, which indicates that EGCG can not only affect the transcription of genes, but also has no effect on the translation of mRNA, so that the expression of LXRα, Idol and LDLR proteins is consistent with the expression of mRNA [4].
LXRα is essential in the adipoietic response, where LXRα is the core of cholesterol homeostasis, and activation of LXRα induces genes involved in cholesterol leakage, blocking LDL-c uptake [21]. LXRα has also been shown to regulate the immune response of macrophages, including those in the liver [3]. As a lipid-driven low-grade chronic inflammatory disease of blood vessel wall, the lipid metabolism and immune regulation regulated by LXRα indicate that LXRα is an important gene influencing the progression of ASCVD [10]. 25-OHC is produced by cholesterol and plays an important role in cholesterol metabolism, and can act as an endogenous LXRα ligand, induce LXRα activation, and then participate in lipid metabolism and inflammatory responses, thereby affecting the ASCVD process [14]. In HepG2 cells treated with 25-OHC and EGCG, the expression of LXRα protein and mRNA could recover the initial expression with the increase of EGCG concentration, which is consistent with the results of Wang et al. [20].
Idol is an E3 ubiquitin ligase that triggers ubiquitination of LDLR and promotes its internalization and degradation due to its unique c-terminal ring domain, which is directly regulated by LXRα [13]. The low-density lipoprotein receptor (LDLR), an intact membrane protein most abundantly expressed in the liver, binds to circulating LDL-c and clears it by endocytosis. Therefore, the expression of LDLR in hepatocytes is inversely correlated with plasma LDL-c levels [4]. In HepG2 cells treated with 25-OHC and EGCG, the expression of Idol and LDLR proteins and mRNA could recover the original expression with the increase of EGCG concentration, which has been confirmed in previous related studies [8]. These results suggest that EGCG also has the effect of improving the expression of Idol and LDLR, thereby improving the lipid metabolism abnormalities and inflammatory response caused by 25-OHC.
Although EGCG has been shown to play an important role in regulating the LXRα-LDLR pathway, and has a significant regulatory effect on the three important proteins in this pathway, both transcription level and protein level, there are still some unelucidated problems in this study. Firstly, we only explored the role of EGCG HepG2 cells. No experiments have been carried out on animals. Secondly, we did not further investigate the interaction between EGCG and LXRα-LDLR axis in hepatocellular carcinoma in vivo. Thirdly, the specific site of EGCG in the LXRα-LDLR pathway is not clear, which still needs to be elucidated and supplemented by a large number of studies. In the next step, we will explore the effect and mechanism of EGCG-LXRα-LDLR in hepatocellular carcinoma by conducting animal experiments.
Nonetheless, this study confirms that EGCG can effectively alleviate abnormal expression in the LXRα-LDLR axis due to 25-OHC at the transcriptional level and protein level.
Data availability
All data generated or analysed during this study are included in this. Further enquiries can be directed to the corresponding author.
Abbreviations
- EGCG:
-
Epigallocatechin gallate
- LDL:
-
Low-density lipoprotein
- HepG2:
-
Human hepatoma cell line
- LDLR:
-
Low-density lipoprotein receptor
- LXRα:
-
Liver X receptor α
- ASCVD:
-
Atherosclerotic cardiovascular disease
- LDL-c:
-
Low-density lipoprotein cholesterol
- AS:
-
Atherosclerosis
- PCSK9:
-
Protein invertase subtilin/ketoin type 9
- Mylip:
-
Myosin regulates light chain interacting protein
- IFN-γ:
-
Interferon γ
- IL-17A:
-
Interleukin-17A
- TCR:
-
T cell receptor
- CVD:
-
Cardiovascular disease
- EC:
-
Epicatechin
- ECG:
-
Epicatechin gallate
- EGC:
-
Epigallocatechin
- EGCG:
-
Epigallocatechin gallate
- 25-OHC:
-
25-Hydroxycholesterol
- FBS:
-
Fetal bovine serum
- SDS:
-
Sodium dodecyl sulfate
- ECL:
-
Enhanced chemiluminescence
- PBS:
-
Phosphate-buffered saline
References
Ataei S, Ganjali S, Banach M, Karimi E, Sahebkar A. The effect of PCSK9 immunization on the hepatic level of microRNAs associated with the PCSK9/LDLR pathway. Arch Med Sci. 2023;19:203–8.
Bernatova I. Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: focus on cardiovascular and neuropsychological health. Biotechnol Adv. 2018;36:666–81.
Bideyan L, Fan W, Kaczor-Urbanowicz KE, Priest C, Casero D, Tontonoz P. Integrative analysis reveals multiple modes of LXR transcriptional regulation in liver. Proc Natl Acad Sci U S A. 2022;119(7):e2122683119.
Bjune K, Wierød L, Naderi S. Triciribine increases LDLR expression and LDL uptake through stabilization of LDLR mRNA. Sci Rep. 2018;8:16174.
Chae HS, Pel P, Cho J, Kim YM, An CY, et al. Identification of neolignans with PCSK9 downregulatory and LDLR upregulatory activities from Penthorum chinense and the potential in cholesterol uptake by transcriptional regulation of LDLR via SREBP2. J Ethnopharmacol. 2021;278:114265.
Chen SF, Chen PY, Hsu HJ, Wu MJ, Yen JH. Xanthohumol suppresses Mylip/Idol gene expression and modulates LDLR abundance and activity in HepG2 cells. J Agric Food Chem. 2017;65:7908–18.
Choi YJ, Lee SJ, Kim HI, Lee HJ, Kang SJ, et al. Platycodin D enhances LDLR expression and LDL uptake via down-regulation of IDOL mRNA in hepatic cells. Sci Rep. 2020;10:19834.
Cui CJ, Jin JL, Guo LN, Sun J, Wu NQ, et al. Beneficial impact of epigallocatechingallate on LDL-C through PCSK9/LDLR pathway by blocking HNF1α and activating FoxO3a. J Transl Med. 2020;18:195.
Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. 2018;210:296–310.
Findeisen HM, Voges VC, Braun LC, Sonnenberg J, Schwarz D, et al. LXRα regulates oxLDL-induced trained immunity in macrophages. Int J Mol Sci. 2022;23(11):6166.
Galimberti F, Casula M, Olmastroni E. Apolipoprotein B compared with low-density lipoprotein cholesterol in the atherosclerotic cardiovascular diseases risk assessment. Pharmacol Res. 2023;195:106873.
Girona J, Rodríguez-Borjabad C, Ibarretxe D, Heras M, Amigo N, et al. Plasma inducible degrader of the LDLR, soluble low-density lipoprotein receptor, and proprotein convertase subtilisin/kexin type 9 levels as potential biomarkers of familial hypercholesterolemia in children. J Clin Lipidol. 2018;12:211–8.
Leitch EK, Elumalai N, Fridén-Saxin M, Dahl G, Wan P, et al. Inhibition of low-density lipoprotein receptor degradation with a cyclic peptide that disrupts the homodimerization of IDOL E3 ubiquitin ligase. Chem Sci. 2018;9:5957–66.
Liu Y, Wei Z, Ma X, Yang X, Chen Y, et al. 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. J Lipid Res. 2018;59:439–51.
Ma M, Hou C, Liu J. Effect of PCSK9 on atherosclerotic cardiovascular diseases and its mechanisms: focus on immune regulation. Front Cardiovasc Med. 2023;10:1148486.
Mortensen MB, Dzaye O, Bøtker HE, Jensen JM, Maeng M, et al. Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the western denmark heart registry. Circulation. 2023;147:1053–63.
Newman CB, Tobert JA. Targeting PCSK9 With antibodies and gene silencing to reduce LDL cholesterol. J Clin Endocrinol Metab. 2023;108:784–90.
Sinning D, Landmesser U. Low-density lipoprotein-cholesterol lowering strategies for prevention of atherosclerotic cardiovascular disease: focus on sirna treatment targeting PCSK9 (Inclisiran). Curr Cardiol Rep. 2020;22:176.
Teramoto M, Yamagishi K, Muraki I, Tamakoshi A, Iso H. Coffee and green tea consumption and cardiovascular disease mortality among people with and without hypertension. J Am Heart Assoc. 2023;12:e026477.
Wang W, Zhang ZZ, Wu Y, Wang RQ, Chen JW, et al. (-)-Epigallocatechin-3-gallate ameliorates atherosclerosis and modulates hepatic lipid metabolic gene expression in apolipoprotein e knockout mice: involvement of TTC39B. Front Pharmacol. 2018;9:195.
Xiong Y, Xu Q, Lin S, Wang Y, Lin Y, Zhu J. Knockdown of LXRα inhibits goat intramuscular preadipocyte differentiation. Int J Mol Sci. 2018;19(10):3037.
Zamani M, Kelishadi MR, Ashtary-Larky D, Amirani N, Goudarzi K, et al. The effects of green tea supplementation on cardiovascular risk factors: a systematic review and meta-analysis. Front Nutr. 2022;9:1084455.
Zanka K, Kawaguchi Y, Okada Y, Nagaoka S. Epigallocatechin gallate induces upregulation of LDL receptor via the 67 kDa laminin receptor-independent pathway in HepG2 cells. Mol Nutr Food Res. 2020;64:e1901036.
Zhou Y, Guo Y, Zhuang X, Du Z. Docosahexanoic acid modifies low-density lipoprotein receptor abundance in HepG2 cells via suppression of the LXRα-Idol pathway. Mol Med Rep. 2015;11:2329–33.
Acknowledgements
Not applicable.
Funding
The authors have not received any funding support.
Author information
Authors and Affiliations
Contributions
FFL is resposible for the definition of intellectual content, literature research, clinical studies, experimental studies, data acquisition & analysis, statistical analysis, manuscript preparation & editing & review; WXH is resposible for the data analysis, statistical analysis; CHY is resposible for the data analysis, statistical analysis; BHY is resposible for the data analysis; ZMD is resposible for the guarantor of integrity of the entire study, study concepts & design, definition of intellectual content, manuscript preparation & editing; QHW is resposible for the literature research, data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no potential conflicts of interest to disclose.
Consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors have read the manuscript and consented to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Somnath Paul; Arpan Dey Bhowmik Pallab Shaw.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, F., Huang, W., Yang, C. et al. Epigallocatechin gallate induces an up-regulation of LDLR accompanied by a reduction of idol in Hepg2 cells. Nucleus (2024). https://doi.org/10.1007/s13237-024-00491-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13237-024-00491-5