Abstract
Background
Survival of preterm infants has improved drastically. In addition to significant contribution to neonatal mortality, impact of prematurity among survivors may continue through life impairing long-term physical life through neuro-disability and increased risk of cerebral palsy. Maternal administration of magnesium sulfate prior to impending preterm birth is an effective strategy to reduce neuromorbidity.
Aim
To investigate the effectiveness of antenatal magnesium sulfate for neuroprotection in preterm infants between 26 and 34 weeks in preventing early neonatal morbidity and mortality. Secondary objective was to assess any adverse events with the use of magnesium sulfate on the mother and neonate.
Method
This was a prospective observational comparative study for 2 years at our tertiary care hospital of 100 pregnant women who gave preterm births. Fifty infants each were born to mothers who were either not given MgSO4 (Group 1) or given 4gm intravenous loading dose MgSO4 (Group 2), preferably 4 h prior to preterm birth.
Results
Among all the preterm in our study, 81% delivered between 30 and 34 weeks. There was no significant difference in terms of maternal mortality or serious morbidity including postpartum hemorrhage, caesarian section rates or length of hospital stay among women receiving MgSO4 versus no MgSO4. Mild maternal side effects secondary to magnesium sulfate were experienced in 8% cases. There were no significant differences between both groups for low 5 min APGAR, need for NICU admission, neonatal convulsions, hyperbilirubinemia, necrotizing enterocolitis, periventricular leukomalacia and septicemia. There was a trend toward reduced risk in the magnesium sulfate group for need for mechanical ventilation and ongoing respiratory support, intraventricular hemorrhage, neonatal hypotension, hypothermia, length of NICU stay. IVH was less frequent and less severe in babies exposed to antenatal MgSO4 (8%) as compared to non-MgSO4 group (16%). Neonatal morbidities were more when antenatal MgSO4 was given less than 4 h from delivery.
Conclusion
MgSO4 is a safe drug to use in antenatal women at risk for impending preterm. Antenatal magnesium sulfate given to women in established preterm labor conferred significant neuroprotective advantage to the neonate. MgSO4 also has protective effect on the need of invasive ventilatory support in preterm infants. Given the breadth of evidence in its favor, it is time for us to start using MgSO4 in clinical practice for neuroprotective intent in all our extreme preterm births.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among 135 million neonates born each year worldwide, almost 14.9 million are preterm representing a preterm birth rate of 11.1% [1]. Approximately 70% of neonatal deaths, 36% of infant deaths and 25–50% of cases of long-term neurological impairment in the USA occur in these preterm live births [2].
With improved prenatal and neonatal care, survival of premature infants has increased significantly. However, these premature infants who survive exert a heavy burden on families, society and the healthcare system. Short-term complications of premature birth include respiratory and cardiovascular complications, intracranial hemorrhage, necrotizing enterocolitis, hypothermia and NICU admissions. Those who emerge from these initial obstacles may suffer from long-term sequelae of intellectual disability, cognitive dysfunction, hearing and visual impairments. These disabilities increase proportionally with decreasing gestational age at birth, lower birthweight and also decreasing quality of medical care facility [3].
Perinatal morbidity and death following preterm birth can be reduced not only by good neonatal care but by timely interventions provided to the mother in the form of antenatal steroids, antibiotics and MgSO4 for improving lung maturity, susceptibility to infections and neuroprotection, respectively [4].
The exact mechanism of the role of magnesium sulfate (MgSO4) in prevention of neuronal injury to fetal brain is unclear. Magnesium ions are involved in intracellular glycolysis, oxidative phosphorylation, protein synthesis and maintenance of cell membrane integrity. There is some evidence that magnesium reduces the production of pro-inflammatory cytokines and free radicles following hypoxic ischemic reperfusion and also prevents calcium-induced injury.
The use of MgSO4 for seizure prevention in patients with pre-eclampsia and as a tocolytic agent in premature labor has been known and widely used for some time. Nelson and Grether were the first to suggest, in a case–control study of extremely low-birthweight infants in 1995, that there existed a link between the incidence of cerebral palsy and exposure to MgSO4. It was serendipitously observed that infants exposed to MgSO4 in pregnancy were less likely to develop cerebral palsy compared with those not exposed, all other factors remaining constant [5].
Since then three randomized trials, one each in Australia and New Zealand (Australia Collaborative Trial of the MgSO4 group/ ACTOMAG), France (PREMAG) and USA (Beneficial effect of antenatal magnesium sulfate / BEAMS) have been conducted to assess the efficacy of MgSO4 in preventing neonatal mortality, perinatal cerebral injury and cerebral palsy in premature births [6,7,8]. Subsequent meta-analysis of all available clinical trials in 2009 confirmed its neuroprotective effect [9, 10]. The Cochrane review in the same year also recommended the use of MgSO4 for neuroprotection when birth is anticipated before 32 weeks of gestation [11].
Although the goal of all the three randomized trials was to evaluate the effect of magnesium sulfate on neurodevelopmental outcomes and mortality in preterm neonates, comparisons between trials are difficult due to differences in population studied, gestational age at treatment, inclusion and exclusion criteria, MgSO4 regimes and outcome variables assessed. MgSO4 regimes have varied from a loading dose of 4gm in 15 min to 6 gm in 20 min with maintenance dose varying from none to 3 gm/ hour and duration of infusion from 12 to 24 h.
On the contrary, few studies on early outcomes among preterm infants treated with MgSO4 for neuroprotective intent have demonstrated increased risk of intraventricular hemorrhage, impaired intestinal blood flow in the few hours after birth, increased spontaneous bowel perforation, increased neonatal intensive care admissions and need for intubation [12].
In spite of the recommendations, drug being inexpensive, clinicians experienced with its use in eclampsia, it is still not being widely practiced, and to my knowledge, there are no known Indian comparative studies on use of magnesium sulfate for neuroprotection.
We decided to use magnesium sulfate for fetal neuroprotection in patients with imminent preterm delivery as a standard of care in our unit in a tertiary care hospital. However, all the other units of our hospital were still not convinced of its efficacy as well as worried about its maternal and neonatal complications. We planned to compare the perinatal outcome in 100 cases. The Institutional Ethics Committee granted approval for the study.
Aims and Objectives
1. To investigate the effectiveness of antenatal magnesium sulfate for neuroprotection in preterm infants between 26 and 34 weeks in preventing early neonatal morbidity and mortality.
2. To assess any adverse events with the use of magnesium sulfate on the mother and neonate.
Methodology
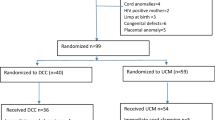
This was a prospective comparative observational study from April 2016 till March 2018 at our tertiary care referral hospital of 100 pregnant women who gave preterm births between 26 and 34 weeks either due to spontaneous preterm labor and / or planned preterm birth for fetal or maternal indications.
Out of these, 50 infants were born to mothers who were not given injection MgSO4 (Group 1) and 50 infants were born to mothers in impending preterm delivery who were given injection MgSO4 (Group 2) 4gm intravenous loading dose in 100 ml normal saline over 30 min preferably 4 h prior to preterm birth as per standard of care. No maintenance dose was given. If delivery was imminent, it was still given irrespective of 4-h window period. We excluded from both groups, women who delivered before they could get the benefit of two doses of steroids for lung maturity, multiple pregnancies and those with a major fetal abnormality.
Pregnant women and neonates were cared for according to standard clinical practice. Outcome of these preterm infants and their mothers were analyzed through information collected from patient record till postnatal 1 month or hospital discharge whichever was later and was used for comparison.
Results
Among the 100 women in our study, average age of the mothers was 28.2 ± 4.74 years with a range of 19–41 years. The average mothers’ age in Group1 was 29.0 ± 4.88 and in Group 2 was 27.3 ± 4.49 [t (98) = 1.747, p = 0.084].
Maximum delivery occurred between 30 and 34 weeks of gestation (n-81, 81%) (Fig. 1). Gestational age at birth was similar in the MgSO4 (31.1 ± 1.73 weeks) and non-MgSO4 group (31.4 ± 1.49 weeks) [t (98) = 1.084, p = 0.281].
Pregnancy-induced hypertension, fetal growth restriction (FGR) and history of previous preterm delivery or second trimester abortion were found to be the major risk factors for preterm delivery. Eighty-three percent of all patients had 1 or more risk factors and only 17% were low-risk pregnancies.
Fifty-two percent patient went into spontaneous preterm labor (n-30) or spontaneous premature rupture of membranes (PROM) (n-22) and 48% were indicated preterm deliveries due to maternal medical disease such as hypertensive disorders, placental causes (placental abruption and previa) and fetal causes (FGR and oligohydramnios). The single most common indication for preterm delivery was abnormal Doppler flows (27%) barring patients in spontaneous labor.
Seventy percent preterms delivered by LSCS (lower segment cesarean section) (Table 1). There were more number of LSCS in non-MgSO4 group (57%) compared to MgSO4 group (42%) which was statistically significant [χ(1) = 4.762, p = 0.029]. The most common indication for LSCS being abnormal Color Doppler 26% followed by PIH 15% and prolonged PPROM 11%.
The average time elapsed between MgSO4 and delivery was 3.74 ± 1.675 h with the maximum time being 6 h and minimum time being 1 h (Fig. 2). Almost equal numbers delivered in less than and more than 4 h ideal protocol (24/26) (Table 2).
Only 4 out of the 50 women (8%) who received MgSO4 had mild side effects, including nausea (1), dizziness (1), flushing (1) and tachycardia (1), which subsided spontaneously after 1- 3 h, none required medications or cessation of MgSO4 therapy. Out of the 50 patients in Group 1 (non-MgSO4), 6 (12%) patients had atonic postpartum hemorrhage (PPH), all of which had cesarean deliveries. Two women who received MgSO4 for neuroprotection had PPH (4%), both of which had vaginal deliveries. Atonic PPH was more seen in non-MgSO4 group; however, it was not statistically significant (p = 0.269). None had estimated blood loss > 1000 ml (Massive postpartum hemorrhage) and all were controlled with uterotonics with no increased morbidity.
The mean birthweight in Group 1 was 1.29 ± 0.346 and in Group 2 was 1.21 ± 0.348 (Table 3) with no statistically significant difference, p = 0.249 (Fig. 3).
Infants with 5 min Apgar score less than 5 were not seen in the MgSO4 group. However, 2 neonates in non-MgSO4 group had 5 min Apgar less than 5 due to cord prolapse in one and extreme prematurity (26 weeks) in the second. The correlation of the effect of MgSO4 on APGAR scores was not significant with almost equal number of neonates with 5 min Apgar less than 7 in both groups (36%vs 38%) [rpb = 0.52, p = 0.611]. The average APGAR score for Group 1 was 7.1 ± 1.54 and for Group 2 was 7.2 ± 1.38.
The methods of resuscitation are summarized in the clustered bar chart (Fig. 4). Thirteen out of 50 neonates (26%) not exposed to antenatal magnesium sulfate required intubation whereas 9 out of 50 infants (18%) exposed to antenatal magnesium sulfate required intubation. Need for CPAP (continuous positive airway pressure) or full ventilation was seen in 24/50 (48%) neonates in non-MgSO4 group and only 16/50 (32%) in the MgSO4 group.
75% (6/8) neonates in non-MgSO4 group who delivered before 30 weeks of gestation required intubation when compared to 47% (8/17) in MgSO4 group. Among neonates who delivered after 30 weeks 16% required intubation in non-MgSO4 when compared to only 3% in MgSO4 group. Twenty-three percent neonates more than 1.5 kg in non-MgSO4 group required intubation compared to MgSO4 group whereas none of the neonates more than 1.5 kg required intubation (Table 4).
Among the 100 live births, 90 neonates required NICU admission. Excluding the 7 infants in which death occurred before discharge, 83 infants were evaluated for the number of days they stayed in the NICU. The average number of days in NICU for infants in Group 1 (non-MgSO4) was 24.1 ± 23.46 days and for Group 2 (MgSO4 group) was 22.1 ± 21.18. There was no statistically significant correlation rpb = 0.44, p = 0.673.
Twenty percent (10/50) neonates not exposed to antenatal MgSO4 required respiratory support for more than 10 days as compared to 12% (6/50) neonates in MgSO4 group. Three neonates not exposed to antenatal MgSO4 required respiratory support for more than 25 days whereas no infants exposed to antenatal MgSO4 required more than 25 days of respiratory support, hence protecting the neonates from adverse effect of excessive oxygenation such as retinopathy of prematurity (Table 5).
Fourteen percent neonates who delivered after 30 weeks of gestation in non-MgSO4 group required ventilator support for more than 10 days when compared MgSO4 group where none of the neonates required ventilator support after 10 days. A point-biserial correlation was run to determine if infants exposed to antenatal MgSO4 required less days on ventilator than controls. The correlation was significant, [rpb = 0.031, p = 0.780]. Infants with antenatal exposure to MgSO4 had more days free of respiratory support in the first 28 days of life. Similar correlation was found with regard to birthweight and need for ventilator support.
The 14 signs and symptoms recorded were analyzed to check whether there were any MgSO4 attributable adverse signs and symptoms. Although we expected hypotension in the neonate given MgSO4 due to transplacental passage of the drug, infants were four times less likely to develop hypotension when MgSO4 was given (χ 2(1) = 6.353, p = 0.012, 95% CI 1.201 to 13.319). Hypothermia (3 from 50 neonates) was also less in infants exposed to antenatal MgSO4 as compared to infants (8 from 50 neonates) not exposed, although not statistically significant (Table 6).
All surviving infants (n-93) underwent a cranial ultrasound within the first 14 days of life. On neurosonogram, 2 infants showed (periventricular leukomalacia) PVL, one each in MgSO4 and non-MgSO4 group and 12 infants had IVH (Intraventricular hemorrhage). IVH was more often seen in non-MgSO4 group (8/12) as compared to MgSO4 group (4/12).
IVH was seen in 9 out of 19 neonates (47%) born before 30 weeks and 3 out of 81 neonates (3.7%) born after 30 weeks of gestation. No IVH was seen in baby weighing more than 1.5 kg.
In the infants not antenatally exposed to MgSO4, 5 neonates delivered before 30 weeks of gestation had IVH and 3 neonates delivered after 30 weeks of gestation also had IVH, which were between 1 and 1.5 kg weight. IVH was seen in only 4 antenatal exposed MgSO4 neonates all of whom were less than 30 weeks of gestation and extremely low birthweight (less than 1 kg) (Table 7).
Of the 4 neonates with IVH in the MgSO4 group, only 1 had grade 2 IVH while 3 had grade 1 IVH, all amenable to resolution. None of the neonates antenatally exposed to MgSO4 had grade 3 or grade 4 IVH, which may have long-term neuromorbidity. Out of 8 neonates with IVH in the non-MgSO4 group, 3 had severe grade 3 IVH and 2 neonates had grade 2 IVH and 3 neonates had grade 1 IVH.
It is seen that all IVH in the MgSO4 exposed group were in pregnancies where MgSO4 was given less than 4 h before delivery where probably the protective effect was inadequate. It may be beneficial to give MgSO4 more than 4 h prior to intention of delivery.
The study found no statistical significant difference in neonatal morbidities like neonatal jaundice and need for phototherapy, septicemia, seizures and necrotizing enterocolitis (NEC) in neonates exposed to antenatal MgSO4 as compared to those not given MgSO4 (Table 8).
While evaluating neonatal outcomes with regard to duration of MgSO4 infusion prior to delivery, 15% infants exposed to antenatal MgSO4 for less than 4 h had seizures whereas no seizures were seen in infants exposed to antenatal MgSO4 for more than 4 h. Eighteen percent infants exposed to antenatal MgSO4 for less than 4 h had NEC whereas no NEC was seen in infants exposed to antenatal MgSO4 for more than 4 h.
Out of 100 preterm neonates studied, there were 7 early neonatal deaths in NICU. Two among the neonatal mortality were in less than 28 weeks of gestation and rest 5 neonatal deaths occurred in 28–30 weeks gestation. All neonatal deaths occurred in extremely low-birthweight babies (less than 1 kg) born at less than 30 weeks maturity.
In the 50 patients in whom MgSO4 was given, there were 5 (10%) NICU deaths as against 2(4%) deaths in the non-MgSO4 group. A Fischer’s Exact test was run which showed that NICU deaths were not significantly associated with MgSO4, p = 0.436.
Discussion
Prevalence of cerebral palsy worldwide has shown a modest increase secondary to increased survival of very low-birthweight infants [13]. White matter injury and intraventricular hemorrhage are the main developmental stage-specific brain disease responsible for these neuro-disabilities [14]. Risk of IVH increases inversely with the gestational age at birth [15].
Although multiple clinical trials and available guidelines from several countries endorse the use of magnesium sulfate for prevention of cerebral palsy in preterm infants [16,17,18], its clinical use for this purpose has undergone limited evaluation outside the context of these trials.
Even in our institution at the time of conducting the study, only our clinical unit was using MgSO4 as a standard of care in women delivering preterm while there was still resistance to its use from all other units. So we decided to undertake this comparative prospective observation study where the obstetric management was performed by the respective consultants, while the NICU and the clinical treatment of the neonates were under a common neonatal team. We planned to compare short-term maternal and perinatal outcomes and safety of use of MgSO4 in routine clinical practice. Given the prospective nature of our study and the strictly defined exposure status and outcomes, information bias was minimized.
From April 2016 to March 2018, 100 cases of preterm birth were studied of which 50 women expecting preterm delivery were given MgSO4 for fetal neuroprotection and 50 women were not given. In all the RCT, different regimens of antenatal MgSO4 have been used for neuroprotection [6, 8, 19]. We have not addressed the issues of different regimens in our study and used the lowest dosage regime which is less likely to lead to maternal side effects.
Baseline maternal characteristics and reasons for preterm birth were similar in both groups. Preterm birth was seen more in the age group of 26–34 years (53%) as that is the most common reproductive age group.
Eighty-one percent of preterm deliveries occurred between 30 and 34 weeks of gestation suggesting the need for concentrating our resources on survival in this age group. However, those that survive in the less than 30 weeks (19%) are the ones at extremely high risk for early and late neonatal morbidity. Steps need to be taken in preventing the burden of neurological sub-normality in these very tiny babies who have braved their way to survival.
Almost equal numbers of preterm delivery were following spontaneous labor (52%) and iatrogenic preterm deliveries in our study that is similar to the National Centre for Health Statistics which also states that preterm labor precedes almost 50% of all preterm births [2]. In the first Australian randomized trial the primary reason for preterm birth was spontaneous labor (63%) and more than half were primigravidas with no risk factors [6]. Since our institution is a tertiary referral center for high-risk pregnancies hence the high numbers of indicated preterm births and few (17%) low-risk pregnancies.
Non-MgSO4 group saw more LSCS (57%) as compared to those given MgSO4 (42%) which was statistically significant. This result was contrary to the three trials [6, 8, 19] which reported no significant difference in mode of birth. Our results may be skewed toward more vaginal delivery in MgSO4 group due to different obstetric teams working in the two groups with our unit (MgSO4 group) protocol of giving a fair trial to all preterm labor.
More patient delivered vaginally in the group where the 4 h could not be completed probably due to preponderance of spontaneous active labor in these patients. Relation of timing of MgSO4 with the mode of delivery was not statistically significant (Table 2).
MgSO4 was given only when the delivery was inevitable or decision for LSCS was taken. None of the patient had a prolongation of gestation due to the tocolytic effect of magnesium sulfate. Four grams MgSO4 in neuroprotective dose used in the Premag trial also failed to prevent preterm labor [19].
Maternal adverse effects from MgSO4 therapy are an important issue in administering antenatal MgSO4. Due to its peripheral vasodilator effect when given intravenously, Magnesium sulfate may cause a sensation of warmth, flushing and sweating. It may also cause side effects like nausea, vomiting, headache, dizziness palpitation, tachycardia, hypotension and hypocalcemia. Intravenous MgSO4 if given too rapidly or in too high dosage may accentuate muscle relaxation of respiratory muscles leading to collapse due to its neuromuscular blocking effect. MgSO4 in above recommended range could lead to severe postpartum hemorrhage, respiratory depression, pulmonary edema and cardiac arrest.
No serious adverse events occurred leading to stoppage of the magnesium sulfate infusion prematurely in our study. Minor maternal side effects were encountered in the three major trials, however no serious maternal complications like cardiac arrest, respiratory failure or death were seen [9,10,11]. Consistent with the above evidence, our study has also shown that MgSO4 can be safely administered to women for neuroprotection as there is no clinically significant effect on maternal morbidity.
The mean birthweight of the infants and the weeks of gestation in the two groups were comparable. Extreme preterm and extreme low-birthweight infants that are principal risk factors for IVH and cerebral palsy in our study constituted about a quarter of the total. The rate of CP in very low-birthweight (VLBW) infants reported in literature is 4–8% [20].
MgSO4 has been used for obstetric indication for many decades. Despite the familiarity and comfort of use, there are concerns about the potential adverse effects of antenatal MgSO4 on preterm infants. Mina Abbassi in a study on neonatal effects of MgSO4 given to 6654 women with pre-eclampsia suggested hypotonia, lower 5 min apgar, intubation and admission to NICU were all significantly increased [21]. Five-minute Apgar score < 7 which quantitates clinical signs of neonatal depression showed no difference between the two groups in our study which is similar to the Cochrane systematic review [11].
It is found in our study that the infants who received antenatal MgSO4 were less likely to require invasive mechanical ventilation on either day 1 or day 3 of life compared to the group not received antenatal MgSO4, despite lower birthweight or lower gestational age. MgSO4 had protective effect on the need of invasive ventilation in preterm infants.
Neonates with antenatal exposure to MgSO4 had more days free of respiratory support in the first 28 days of life hence protecting the neonates from adverse effect of excessive oxygenation such as retinopathy of prematurity. Additionally, the beneficial effect of antenatal treatment was uniform between those recruited between 30 and 34 weeks and also those less than 30 weeks. Thus, it appears that the treatment should not be restricted to only the latter.
MagNET trial raised some concerns about MgSO causing harm to the neonates as their analysis showed more adverse events in infants whose mothers received MgSO4 (32%) compared with those where mothers received placebo (19%) although not statistically significant [22]. A Binomial Logistic Regression was performed in our study to ascertain the effects of MgSO4 on 14 neonatal signs and symptoms. Hypotension and hypothermia were significantly less with MgSO4 exposed neonates. MgSO4 exposed neonates were more likely to have poor sucking reflex than their non-MgSO4 counterparts although not statistical significant.
Agustín Conde-Agudelo in his meta-analysis suggested a non-significant but increased risk of necrotizing enterocolitis in the MgSO4 group [10]. Another recent publication by Rattray also reported possible association between antenatal exposure to MgSO4 in extremely low-birthweight infants less than 25 weeks and spontaneous intestinal perforation [23]. Among the 14 infants in our NICU who had NEC, our study demonstrated no association between MgSO4 and NEC (7 vs 7) although we had no babies < 25 weeks in our inclusion criteria.
Prevention of IVH is of prime importance in preterm infants as it is associated with neuro-disability, death and cerebral palsy. Risk of IVH increases with lower gestational age at birth as seen in our study as well. Neonates in our study who developed IVH were significantly more in non-MgSO4 group (8/50, 16%) as compared to MgSO4 group (4/50, 8%). IVH in the neonates born after 30 weeks was only seen in the non-MgSO4 group. Additional protection was seen with regard to the severity of the IVH in our MgSO4 exposed neonates.
Kuban and colleagues in 1992 and later Van de Bor et al. and Levitron noted a decrease in incidence of intraventricular hemorrhage in very low-birthweight infants born to pre-eclamptic women who received MgSO4 [24,25,26]. Crowther 2003 and Rouse 2008 RCT also suggested a similar protective effect as was seen in our study. However, they conjectured that perhaps the protective effect of MgSO4 is beneficial only in neonates of earlier gestational age [6, 8]. Our study suggests significant benefit of MgSO4 for both 30–34 weeks and < 30 weeks with regard to IVH.
The presumed mechanism of action of MgSO4 for neuroprotection is dependent on adequate fetal levels of magnesium at the time of delivery. Animal studies evaluating placental transfer of magnesium sulfate have shown that it crosses fetal blood brain barrier within 2 h of sustained maternal infusion but its concentrations increase in the forebrain only after 4 h of treatment [27]. All neonatal outcomes that is 5 min Apgar score, need for resuscitation, hypotension, sepsis, seizures, IVH, NEC and number of days in NICU were better when time interval between MgSO4 and delivery was more than 4 h in our study. With this evidence, one should aim to commence MgSO4 at least 4 h prior to delivery. However, where it is not possible to achieve the 4-h window period, MgSO4 should still be administered, as it is likely to show some benefit.
Infant death during hospitalization was higher in the MgSO4 group [5] as compared to control [2] although not statistically significant. All the 7 neonatal deaths whether exposed or not exposed to antenatal MgSO4, were extremely premature and extremely low birthweight and hence the detrimental outcomes on neonates may be difficult to attribute directly to the effects of MgSO4. While Crowther, Magpie, Marret and Rouse showed no significant mortality difference between infants exposed to antenatal MgSO4 and infants not exposed to antenatal MgSO4, Mittendorf showed significant more death in MgSO4 group than non-MgSO4 group [6,7,8, 22, 28]. The neonatal mortality in our study was lesser with increasing gestational age and birthweight with no death in infants more than 1 kg.
Conclusion
MgSO4 is a safe drug to use in antenatal women at risk for impending preterm birth. There is no significant difference in terms of maternal mortality or serious morbidity among women receiving MgSO4 versus no MgSO4. Additionally, no significant increase in postpartum hemorrhage, caesarian section rates or length of hospital stay is expected in women who receive MgSO4. Timing of MgSO4 had no significant association with the mode of delivery.
No significant difference regarding neonatal 5 min APGAR, convulsions, hyperbilirubinemia, necrotizing enterocolitis, periventricular leukomalacia, septicemia and need for NICU admission is seen with antenatal MgSO4 use.
A trend toward reduced risk for invasive mechanical ventilation on day 1 of life, requirement for ongoing respiratory support, intraventricular hemorrhage, neonatal hypotension and hypothermia and length of hospital stay are additional beneficial effects of antenatal MgSO4.
Prenatal MgSO4 was protective with regard to frequency and severity of neonatal IVH both before 30 weeks and after 30 weeks of gestation.
Intraventricular hemorrhage, 5 min Apgar score less than 7, need for resuscitation, neonatal seizures and other neonatal morbidities were seen more in infants exposed to antenatal MgSO4 for less than 4 h as compared to more than 4 h. It may be beneficial to give MgSO4, at least 4 h prior to intention to delivery. However, benefit of antenatal MgSO4 should not be denied to women where anticipated birth is earlier and the 4 h gap is not available.
Limitations
Firstly, only a small number of cases were studied. Larger cases are required to document a significant reduction in adverse outcome with magnesium therapy and to assess the neuroprotective role of injection MgSO4 in neonatal outcomes.
Secondly, because of limited period of study and other practical constraints, the babies were not followed up, to document the long-term effects of such therapy. Controlled trials with larger number of babies with follow-up for defined period to document long-term neurodevelopmental outcome is required.
Among pregnant women at risk of imminent preterm birth between 26 and 34 weeks, magnesium sulfate therapy is effective in protecting the fetus from neurological complications and is safe both for the mother and the neonate. Magnesium sulfate for neuroprotective intent should only be given if preterm birth is likely within the next 24 h. Minimum dosage of 4gm IV in infusion over 30 min may be given even if delivery is imminent and 4 h are not available.
In spite of various recommendations and guidelines, drug being inexpensive, clinicians vast experience with its use in eclampsia, it is still not being widely practiced in India. With our Indian study of comparison between use and non-use of MgSO4 in women with preterm birth between 26 and 34 weeks for neuroprotection in surviving infants showing promising results, we anticipate widespread use of antenatal MgSO4 in our country. Magnesium sulfate can protect the developing fetal brain and so has significant potential to reduce disability.
References
Blencowe H, Cousens S, Oestergaard MZ, Chou D, et al. National, regional and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72.
MacDorman MF, Callaghan WM, Mathews TJ et al. Trends in preterm related infant mortality by race and ethnicity: United States, 1999–2004. NCHS Health E stat. Hyattsville (MD): National Centre for Health Statistics; 2007. http://www.cdc.gov/nchs/data/hestat/infantmort99-04/infantmort99-04.htm.
Woods NS, Marlow N, Costeloe K et al. Neurological and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000; 343:378–84.
Iams JD, Romero R, Culhane JF, et al. Primary, secondary and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75.
Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birth weight infants? Paediatrics. 1995;95:263–92.
Crowther CA, Hiller JE, Doyles LW, et al. Australasian Collaborative Trial of Magnesium Sulfate (ACTOMgSO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA 2003;290: 2669–76.
Marret S, Marpeau L, Follet-Bouhamed C, et al. Effect of magnesium sulfate on mortality and neurological morbidity of the very preterm newborn (less than 33 weeks) with 2 year neurological outcomes: results of the prospective PREMAG trial. Gynaecologie Obstetrique and fertilite. 2008;36:278–88.
Rouse DJ, Hirtz DG, Thom E, et al. A randomized controlled trial of magnesium sulfate for prevention of cerebral palsy. N Engl J Med. 2008;359:895–905.
Costantine MM, Weiner SJ. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: A meta-analysis for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Obstet Gynecol. 2009;114:354–64.
Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks gestation: a systematic review and meta analysis. Am J Obstet Gynecol. 2009;200:595–609.
Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulfate for women at risk of preterm birth for neuroprotection of fetus. Cochrane database of systematic reviews 2009: CD004661.
Amiri S, Soleimani F, Alavi MH. Correlation between anthropometric indices at birth and developmental delay in children aged 4–60 months in Iran. Int J Gen Med. 2012;5:683–7.
Moster D, Lie RT, Markestad T. Long term medical and social consequence of preterm birth. N Engl J Med. 2008;359:262–73.
Marlow N. Neurocognitive outcome after very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2004;89:F224–8.
Vohr B, Allan WC, Scott DT, et al. Early onset intraventricular hemorrhage in preterm neonate: incidence of neurodevelopment handicap. Semin Perinatol. 1999;23:212–7.
Magee L, Sawchuck D, Synnes A, et al. SOGC Clinical Practice Guideline: magnesium sulfate for fetal neuroprotection. J Obstet Gynecol Can. 2011;33:516–29.
American College of Obstetricians and Gynecologists, Society for Maternal Fetal Medicine Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115:669–71.
Royal College of Obstetricians and Gynecologists. Magnesium sulfate to prevent cerebral palsy following preterm birth: Scientific impact paper no. 29.2011.
Marret S, Maarpeau L, Zupan-Simunek V et al. PREMAG trial group. Magnesium sulfate given before very preterm birth to protect infant brain: the randomized controlled PREMAG trial. BJOG. 2007;114:310–18.
Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487–91.
Abbassi-Ghanavati M, James MA, Donald DM, et al. Neonatal effect of Magnesium sulfate given to the Mother. Am J Perinatol. 2012;29(10):795–800.
Mittendorf R, Dambrosia J, Pryde PG, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–8.
Manijeh K, Erin ASC, Bradley AY, et al. Antenatal magnesium sulfate, Necrotizing Enterocolitis and Death among neonates < 28 weeks gestation. AJP Rep. 2016;6(1):148–54.
Kuban KC, Leviton A, Pagano M, et al. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage. J O Child Neurol.;7:70–6.
Van de Bor M, Verloove-Vanhorick SP, Brand R, et al. Incidence and prediction of periventricular–intraventricular hemorrhage in very preterm infants. J Perinat Med. 1987;15:333–9.
Levinton A, Kuban KC, Pagano M, et al. Maternal toxemia and neonatal germinal matrix hemorrhage in intubated infants less than 1751g. Obstet Gynecol. 1988;72:571–6.
Hallak M, Cotton DB. Transfer of maternally administered magnesium sulfate into fetal compartment of rat: assessment of amniotic fluid, blood and brain concentration. Am J Obstet Gynecol.;169:427.
Magpie Trial Follow-up Study Collaborative Group The Magpie Trial: a randomized trial comparing magnesium sulfate with placebo for preeclampsia. Outcome for children at 18 months. BJOG. 2007;114:289–99.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Human and animal rights
Research involves human participants but since it is a prospective observational study, there has been no direct risk to participants. Study has been approved by the hospital ethics committee.
Informed consent
Informed consent has been taken from all participants. Informed consent document (ICD) has been attached at the end of the manuscript as annexure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Vandana Bansal is Associate Professor, Department of Obstetrics and Gynaecology, Nowrosjee Wadia Maternity Hospital, Seth G.S. Medical College, Parel, Mumbai, Maharashtra, India; Dr. Avinash Desai is Consultant, Department of Neonatology, Nowrosjee Wadia Maternity Hospital, Parel, Mumbai, Maharashtra, India.
Rights and permissions
About this article
Cite this article
Bansal, V., Desai, A. Efficacy of Antenatal Magnesium Sulfate for Neuroprotection in Extreme Prematurity: A Comparative Observational Study. J Obstet Gynecol India 72 (Suppl 1), 36–47 (2022). https://doi.org/10.1007/s13224-021-01531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-021-01531-9