Abstract
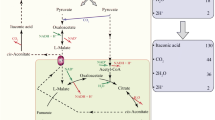
The metabolic responses of Aspergillus terreus NRRL1960 to stress conditions (low dissolved oxygen and pH with limited nitrogen and phosphate) in the two-phase fermentation were investigated in this study. The fermentation kinetics suggested that itaconate production was suppressed under low dissolved oxygen (DO) concentrations. A slight change in pH caused a significant change in itaconate production. The transcriptomic data revealed that under low DO concentration, the glycolytic pathway was uncoupled from the oxidative phosphorylation, resulting in the activation of substrate-level phosphorylation as an alternative route for ATP regeneration. The downregulation of pdh genes, the genes encoding ATP synthase and succinate dehydrogenase, confirmed the observation of the uncoupling of the oxidative TCA cycle from glycolysis. It was found that the upregulation of pyc resulted in a large pool of oxaloacetate in the cytosol. This induced the conversion of oxaloacetate to malate. The upregulation of the gene encoding fumarate hydratase with the subsequent formation of fumarate was found to be responsible for the regeneration of NADPH and ATP under the condition of a low dissolved oxygen level. The large pool of oxaloacetate drove itaconic acid production also via the oxidative TCA cycle. Nevertheless, the downregulation of ATP synthase genes resulted in the deficiency of the proton-pumping H+ ATPase and the subsequent stress due to the failure to maintain the physiological pH. This resulted in itaconate production at a low titer. The fermentation kinetics and the transcriptomic data provided in this study can be used for further process optimization and control to improve itaconate production performance.





Similar content being viewed by others
References
Baykov AA, Evtushenko OA, Avaeva SM (1988) A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem 171:266–270
Birren BW, Lander ES, Galagan JE, et al (2005) Annotation of the Aspergillus terreus NIH264 genome. EMBL/GenBank/DDBJ databases
Boer VM, Crutchfield CA, Bradley PH, Botstein D, Rabinowitz JD (2010) Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol Biol Cell 21:198–211
Boruta T, Bizukojc M (2016) Induction of secondary metabolism of Aspergillus terreus ATCC 20542 in the batch bioreactor cultures. Appl Microbiol Biotechnol 100:3009–3022
Corte-Real M, Leao C (1990) Transport of malic acid and other carboxylic acids in the yeast Hansenula anomala. Appl Environ Microbiol 56:1109–1113
Gyamerah MH (1995a) Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL1960. Appl Microbiol Biotechnol 44:20–26
Gyamerah MH (1995b) Factors affecting the growth form of Aspergillus terreus NRRL1960 in relation to itaconic acid fermentation. Appl Microbiol Biotechnol 44:356–361
Hajjaj H, Niederberger P, Duboc P (2001) Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium. Appl Environ Microbiol 67:2596–2602
Hartman T, Weinrick B, Vilcheze C, Berney M, Tufariello J, Cook GM, Jacobs WR Jr (2014) Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog 10:1–15
Hevekerl A, Kuenz A, Vorlop KD (2014a) Filamentous fungi in microtiter plates-an easy way to optimize itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol 98:6983–6989
Hevekerl A, Kuenz A, Vorlop KD (2014b) Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol 98:10005–10012
Hossain AH, Li A, Brickwedde A, Wilms L, Caspers M, Overkamp K, Punt PJ (2016) Rewiring a secondary metabolite pathway toward itaconic acid production in Aspergillus niger. Microb Cell Factories 15:1–15
Jimenez-Quero A, Pollet E, Zhao M, Marchioni E, Averous L, Phalip V (2016) Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J Microbiol Biotechnol 26:1557–1565
Karaffa L, Diaz R, Papp B, Fekete E, Sandor E, Kubicek CP (2015) A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl Microbiol Biotechnol 99:7937–7944
Kenealy W, Zaady E, Dupreez JC, Stieglitz B, Goldberg I (1986) Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl Environ Microbiol 52:128–133
Klement T, Buchs J (2013) Itaconic acid–a biotechnological process in change. Bioresour Technol 135:422–431
Kocabas A, Ogel ZB, Bakir U (2014) Xylanase and itaconic acid production by Aspergillus terreus NRRL 1960 within a biorefinery concept. Ann Microbiol 64:75–84
Krull S, Hevekerl A, Kuenz A, Prube U (2017) Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biotechnol 101:4063–4072
Kuenz A, Gallenmuller T, Willke T, Vorlop KD (2012) Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol 96:1209–1216
Liu J, Gao Q, Xu N, Liu L (2013) Genome-scale reconstruction and in silico analysis of Aspergillus terreus metabolism. Mol BioSyst 9:1939–1948
Nazir A, Soni R, Saini HS, Kaur A, Chadha BS (2010) Profiling differential expression of cellulases and metabolite footprints in Aspergillus terreus. Appl Biochem Biotechnol 162:538–547
Papagianni M, Wayman F, Mattey M (2005) Fate and role of ammonium ions during fermentation of citric acid by Aspergillus niger. Appl Environ Microbiol 71:7178–7186
Patel MS, Nemeria NS, Furey W, Jordan F (2014) The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem 289:16615–16622
Pimtong V, Ounaeb S, Thitiprasert S, Tolieng V, Sooksai S, Boonsombat R, Tanasupawat S, Assabumrungrat S, Thongchul N (2017) Enhanced effectiveness of Rhizopus oryzae by immobilization in a static bed fermentor for L-lactic acid production. Process Biochem 52:44–52
Protein knowledgebase (UniProtKB) of Aspergillus terreus NIH2624. Available from www.uniprot.org/uniprot/?query=A+terreus+&sort=score. Accessed March 13, 2017
Riscaldati E, Moresi M, Federici F, Petruccioli M (2000) Effect of pH and stirring rate on itaconate production by Aspergillus terreus. J Biotechnol 83:219–230
Roa Engel CA, Straathof AJJ, Zijlmans TW, van Gulik WM, van der Wielen LAM (2008) Fumaric acid production by fermentation. Appl Microbiol Biotechnol 78:379–389
Roa Engel CA, van Gulik WM, Marang L, van der Wielen LAM, Straathof AJJ (2011) Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzym Microb Technol 48:39–47
Romano AH, Bright MM, Scott WE (1967) Mechanism of fumaric acid accumulation in Rhizopus nigricans. J Biotechnol 93:600–604
Schimmel TG (1998) Effect of butyrolactone I on the producing fungus, Aspergillus terreus. Appl Environ Microbiol 64:3707–3712
Songserm P, Thitiprasert S, Tolieng V, Piluk J, Tanasupawat S, Assabumrungrat S, Yang ST, Karnchanatat A, Thongchul N (2015) Regulating pyruvate carboxylase in the living culture of Aspergillus terreus NRRL1960 by L-aspartate for enhanced itaconic acid production. Appl Biochem Biotechnol 177:595–609
Thitiprasert S, Pimtong V, Kodama K, Sooksai S, Tanasupawat S, Assabumrungrat S, Tolieng V, Thongchul N (2016) Correlative effect of dissolved oxygen and key enzyme inhibitors responsible for L-lactate production by immobilized Rhizopus oryzae NRRL395 cultivated in a static bed bioreactor. Process Biochem 51:204–212
Tzollas NM, Zachariadis GA, Anthemidis AN, Stratis JA (2010) A new approach to indophenol blue method for determination of ammonium in geothermal waters with high mineral content. Int J Environ Anal Chem 90:115–126
Wynn JP, Hamid AA, Li Y, Ratledge C (2001) Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpine. Microbiology 147:2857–2864
Xu Q, Li S, Huang H, Wen J (2012) Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol Adv 30:1685–1696
Acknowledgements
The authors would like to thank Dr. Kaemwich Jantama for his fruitful discussion on transcriptomic analysis. The work in RNA-seq gene expression analysis performed by D. Ashley Hill and her colleagues at ResourcePath, Sterlin, VA, USA are highly appreciated. The authors also thank Timothy Wesselman and Roshni Patel from OnRamp Bioinformatics, Inc. (San Diego, CA, USA) for their outstanding work on analyzing the RNA-seq data and differential gene expression analysis.
Funding
Partial support from the Grant for International Research Integration: Research Pyramid, Ratchadapiseksomphot Endowment Fund (GCURP_58_01_33_01) and Thailand Research Fund via the Distinguished Research Professor Grant (DPG5880003) are also acknowledged. The research facility support from the Chulalongkorn Academic Advancement into its 2nd Century Project (CUAASC) is highly appreciated. PS is the recipient of the Royal Jubilee Scholarship Program, Thailand Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Table S1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Songserm, P., Karnchanatat, A., Thitiprasert, S. et al. Metabolic responses of Aspergillus terreus under low dissolved oxygen and pH levels. Ann Microbiol 68, 195–205 (2018). https://doi.org/10.1007/s13213-018-1330-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-018-1330-6