Abstract
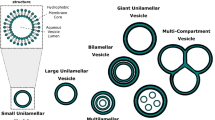
Artificial cell membranes, emulating biological membranes, have been used to elucidate the physiological functions of cells and realize biological applications. Advanced methodologies, along with considerations of biological/functional aspects, have enabled the development of various forms/types of artificial cell membranes commonly based on a lipid bilayer. Previous review articles have extensively explored 2-dimensional membranes but lack consideration on 3-dimensional membranes, which exhibit many advantages for biological platforms, such as large surface area, high stability, and ease of observation. Indeed, 3-dimensional membranes can accommodate a higher population of membrane proteins and show a more sensitive response to analytes than planar membranes. This review highlights the developments of artificial cell membranes in terms of structures and fabrication strategies, with a focus on 3-dimensional free-standing lipid membranes, and concludes with remarks on current key issues and challenges to evolve artificial cell membranes to another level.

Copyright 2006, Cell Press. d Schematic drawing of a BLM produced on a microaperture of a silicon chip, in which the aperture was tapered and treated with a silane coupling agent. Reprinted with permission from [43]. Copyright 2018, American Chemical Society. e Illustration of a droplet interface bilayer (DIB). f Solvent-free BLM formed by bursting giant unilamellar vesicles (GUVs) on a microfabricated aperture. g Schematics of formation steps of BLM arrays in a microfluidic channel (left) and the resulting BLM arrays encapsulating green fluorescence dye, Alexa 488, formed on microchambers with 4 μm in diameter (right). Reprinted with permission from [58]. Copyright 2014, Nature Publishing Group

Copyright 2003, American Chemical Society. b Schematic diagram of receptor-ligand pair based immobilization mechanism of a single vesicle to a substrate (left), where biotin ligands on the vesicle strongly bind to streptavidin patterned on the substrate, and fluorescence images of high-density arrays (every 800 nm, ~ 106 mm−2) of single vesicles labeled with rhodamine (red) and oregon488 (green) (right). Reproduced with permission from [71]. Copyright 2003, Wiley. c Schematic drawing of a microfluidic platform for vesicle formation, trapping, and drug testing, with fluorescence images of produced vesicles for A and trapped vesicles for B in the inset. Reproduced with permission from [73]. Copyright 2019, The Royal Society of Chemistry

Copyright 2011, IEEE. b Illustration of hydrogel stamping of lipids/proteins on ITO substrate and electroformation of giant proteoliposomes from lipid/protein films (left) and corresponding fluorescence images of the lipid/protein films and the proteoliposomes produced by electroformation (right). Reproduced with permission from [34]. Copyright 2013, Wiley. c Schemes of formation of GUVs on a homogeneous hydrogel film (top left) and on a micropatterned hydrogel film (top right), and fluorescence image of an array of GUVs formed on a micropatterned hydrogel with the highly magnified image in the inset (bottom). Reproduced with permission from [77]. Copyright 2019, American Chemical Society

Copyright 2019, Wiley. b Geometry (left) and net charge distribution (right) of 3DFLBs: i) at low frequencies, 3DFLBs are squeezed by the radial Maxwell stress (TMW) or pressure (PMW) arising from the tangential electric field, hence 3DFLBs adopt a prolate shape, ii) and iii) at intermediate frequencies, due to the difference in the conductivity conditions, the net charges across the membrane, illustrated with pluses and minuses, differ depending on the values of the conductivity inside (λin) and outside (λout) of the membrane. The forces applied to the charges by the normal and the tangential electric fields deforms 3DFLBs into a prolate for λin > λex ii) and an oblate for λin < λex iii), and iv) at high frequencies, the electric charges cannot follow the oscillations of the electric field, leading to relax the shape of 3DFLBs into spherical. The green arrows indicate the change of cylindrical lipid structure caused by the morphology changes of semispherical lipid structures owing to energy minimization in lipid membrane surface. c Cross-sectional confocal fluorescence images of 3DFLBs in various shapes generated with different frequencies (left) and comparison of fluorescence intensities of unilamellar and multilamellar 3DFLBs to confirm the relation between lamellarity and sealing property (right) with the corresponding fluorescence images of 3DFLBs in sealing and leaky modes in the inset. Reproduced with permission from [36]. Copyright 2018, American Chemical Society

Copyright 2019, Elsevier
Similar content being viewed by others
References
Komiya, M., et al.: Advances in artificial cell membrane systems as a platform for reconstituting ion channels. Chem Rec. 20, 730–742 (2020)
Xu, C., Hu, S., Chen, X.: Artificial cells: from basic science to applications. Mater Today 19, 516–532 (2016)
Deller, R.C., et al.: Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation. Nat Commun 10, 1887 (2019)
Saier, M.H.: A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev 64, 354–411 (2000)
de la Serna, J.B., Schütz, G.J., Eggeling, C., Cebecauer, M.: There is no simple model of the plasma membrane organization. Front Cell Dev Biol. 4, 1–17 (2016)
Bansod, B., Kumar, T., Thakur, R., Rana, S., Singh, I.: A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94, 443–455 (2017)
Peetla, C., Stine, A., Labhasetwar, V.: Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol Pharm 6, 1264–1276 (2009)
Kalepu, S., Manthina, M., Padavala, V.: Oral lipid-based drug delivery systems–an overview. Acta Pharm Sin B 3, 361–372 (2013)
Howorka, S.: Building membrane nanopores. Nat Nanotechnol 12, 619–630 (2017)
Noakes, M.T., et al.: Increasing the accuracy of nanopore DNA sequencing using a time-varying cross membrane voltage. Nat Biotechnol 37, 651–656 (2019)
Nikoleli, G.-P., Siontorou, C.G., Nikolelis, M.-T., Bratakou, S., Bendos, D.K.: Recent lipid membrane-based biosensing platforms. Appl Sci 9, 1745 (2019)
Bally, M., et al.: Liposome and lipid bilayer arrays towards biosensing applications. Small 6, 2481–2497 (2010)
Wang, Y., et al.: An integrated digital microfluidic bioreactor for fully automatic screening of microalgal growth and stress-induced lipid accumulation. Biotechnol Bioeng 118, 294–304 (2021)
Castellana, E.T., Cremer, P.S.: Solid supported lipid bilayers: From biophysical studies to sensor design. Surf Sci Rep 61, 429–444 (2006)
Yoon, B.K., et al.: Lipid bilayer coatings for rapid enzyme-linked immunosorbent assay. Appl Mater Today 24, 101128 (2021)
Svedhem, S., et al.: In situ peptide-modified supported lipid bilayers for controlled cell attachment. Langmuir 19, 6730–6736 (2003)
Savarala, S., Ahmed, S., Ilies, M.A., Wunder, S.L.: Stabilization of soft lipid colloids: competing effects of nanoparticle decoration and supported lipid bilayer formation. ACS Nano 5, 2619–2628 (2011)
Berti, D., Caminati, G., Baglioni, P.: Functional liposomes and supported lipid bilayers: towards the complexity of biological archetypes. Phys Chem Chem Phys 13, 8769 (2011)
Hirano-Iwata, A., Ishinari, Y., Yamamoto, H., Niwano, M.: Micro-and nano-technologies for lipid bilayer–based ion-channel functional assays. Chem An Asian J 10, 1266–1274 (2015)
Hirano-Iwata, A., et al.: Free-standing lipid bilayers in silicon chips−membrane stabilization based on microfabricated apertures with a nanometer-scale smoothness. Langmuir 26, 1949–1952 (2010)
Hirano-Iwata, A., Taira, T., Oshima, A., Kimura, Y., Niwano, M.: Improved stability of free-standing lipid bilayers based on nanoporous alumina films. Appl Phys Lett 96, 213706 (2010)
Reimhult, E., Kumar, K.: Membrane biosensor platforms using nano-and microporous supports. Trends Biotechnol. 26, 82–89 (2008)
Mazur, F., Bally, M., Städler, B., Chandrawati, R.: Liposomes and lipid bilayers in biosensors. Adv Colloid Interface Sci 249, 88–99 (2017)
Girard, P., et al.: A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys J 87, 419–429 (2004)
Jørgensen, I.L., Kemmer, G.C., Pomorski, T.G.: Membrane protein reconstitution into giant unilamellar vesicles: a review on current techniques. Eur Biophys J 46, 103–119 (2017)
Akashi, K., Miyata, H., Itoh, H., Kinosita, K.: Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys J 71, 3242–3250 (1996)
Ong, S., Chitneni, M., Lee, K., Ming, L., Yuen, K.: Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics 8, 36 (2016)
Shum, H.C., Lee, D., Yoon, I., Kodger, T., Weitz, D.A.: Double emulsion templated monodisperse phospholipid vesicles. Langmuir 24, 7651–7653 (2008)
Le Berre, M., Yamada, A., Reck, L., Chen, Y., Baigl, D.: Electroformation of giant phospholipid vesicles on a silicon substrate: advantages of controllable surface properties. Langmuir 24, 2643–2649 (2008)
Motta, I., et al.: Formation of giant unilamellar proteo-liposomes by osmotic shock. Langmuir 31, 7091–7099 (2015)
Luciani, P., et al.: Receptor-independent modulation of reconstituted Galpha(i) protein mediated by liposomes. Mol. Biosyst. 5, 356–367 (2009)
Rigaud, J.-L., Pitard, B., Levy, D.: Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta-Bioenerg. 1231, 223–246 (1995)
Osaki, T., Kuribayashi-Shigetomi, K., Kawano, R., Sasaki, H., Takeuchi, S. 2011 Uniformly-sized giant liposome formation with gentle hydration. in Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS) 103–106 (IEEE). https://doi.org/10.1109/MEMSYS.2011.5734372
Kang, Y.J., Wostein, H.S., Majd, S.: A Simple and versatile method for the formation of arrays of giant vesicles with controlled size and composition. Adv. Mater. 25, 6834–6838 (2013)
Kamiya, K. et al. 2011 Selective lipid-patterning for heterologous giant liposome array. 15th Int Conf Miniaturized Syst Chem Life Sci. 1137–1139
Kang, D.-H., Han, W.B., Choi, N., Kim, Y.-J., Kim, T.S.: tightly sealed 3D lipid structure monolithically generated on transparent su-8 microwell arrays for biosensor applications. ACS Appl Mater Interfaces 10(47), 40401–40410 (2018). https://doi.org/10.1021/acsami.8b13458
Mueller, P., Rudin, D.O., Ti Tien, H., Wescott, W.C.: Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194, 979–980 (1962)
Montal, M., Mueller, P.: Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A 69, 3561–3566 (1972)
Zagnoni, M., Sandison, M.E., Morgan, H.: Microfluidic array platform for simultaneous lipid bilayer membrane formation. Biosens Bioelectron. 24, 1235–1240 (2009)
Mayer, M., Kriebel, J.K., Tosteson, M.T., Whitesides, G.M.: Microfabricated teflon membranes for low-noise recordings of ion channels in planar lipid bilayers. Biophys J 85, 2684–2695 (2003)
Schmitt, E.K., Vrouenraets, M., Steinem, C.: Channel activity of OmpF monitored in nano-BLMs. Biophys J 91, 2163–2171 (2006)
Römer, W., Steinem, C.: Impedance analysis and single-channel recordings on nano-black lipid membranes based on porous alumina. Biophys. J. 86, 955–965 (2004)
Yamaura, D., et al.: Amphiphobic septa enhance the mechanical stability of free-standing bilayer lipid membranes. Langmuir 34, 5615–5622 (2018)
Eray, M., et al.: Highly stable bilayer lipid membranes (BLMs) formed on microfabricated polyimide apertures. Biosens. Bioelectron. 9, 343–351 (1994)
Oshima, A., et al.: Reconstitution of human ether-a-go-go -related gene channels in microfabricated silicon chips. Anal Chem 85, 4363–4369 (2013)
Syeda, R., Holden, M.A., Hwang, W.L., Bayley, H.: Screening blockers against a potassium channel with a droplet interface bilayer array. J Am Chem Soc 130, 15543–15548 (2008)
Kawano, R., et al.: Automated parallel recordings of topologically identified single ion channels. Sci Rep 3, 1995 (2013)
Poulos, J.L., et al.: Ion channel and toxin measurement using a high throughput lipid membrane platform. Biosens Bioelectron 24, 1806–1810 (2009)
Holden, M.A., Needham, D., Bayley, H.: Functional bionetworks from nanoliter water droplets. J Am Chem Soc 129, 8650–8655 (2007)
Batishchev, O.V., Indenbom, A.V.: Alkylated glass partition allows formation of solvent-free lipid bilayer by montal-mueller technique. Bioelectrochemistry 74, 22–25 (2008)
Schmidt, C., Mayer, M., Vogel, H.: A chip-based biosensor for the functional analysis of single ion channels. Angew Chemie 39, 3137–3140 (2000)
Kresák, S., Hianik, T., Naumann, R.L.C.: Giga-seal solvent-free bilayer lipid membranes: from single nanopores to nanopore arrays. Soft Matter 5, 4021 (2009)
Sondermann, M., George, M., Fertig, N., Behrends, J.C.: High-resolution electrophysiology on a chip: transient dynamics of alamethicin channel formation. Biochim Biophys Acta-Biomembr 1758, 545–551 (2006)
Kreir, M., Farre, C., Beckler, M., George, M., Fertig, N.: Rapid screening of membrane protein activity: electrophysiological analysis of OmpF reconstituted in proteoliposomes. Lab Chip 8, 587 (2008)
Le Pioufle, B., Suzuki, H., Tabata, K.V., Noji, H., Takeuchi, S.: Lipid bilayer microarray for parallel recording of transmembrane ion currents. Anal Chem 80, 328–332 (2007)
Suzuki, H., Pioufle, B.L., Takeuhci, S.: Ninety-six-well planar lipid bilayer chip for ion channel recording fabricated by hybrid stereolithography. Biomed Microdevices 11, 17–22 (2009)
Hirano-Iwata, A., Nasu, T., Oshima, A., Kimura, Y., Niwano, M.: Lipid bilayer array for simultaneous recording of ion channel activities. Appl. Phys Lett 101, 023702 (2012)
Watanabe, R., et al.: Arrayed lipid bilayer chambers allow single-molecule analysis of membrane transporter activity. Nat Commun 51(1), 1–8 (2014)
Baaken, G., Ankri, N., Schuler, A.K., Rühe, J., Behrends, J.C.: Nanopore-based single-molecule mass spectrometry on a lipid membrane microarray. ACS Nano 5, 8080–8088 (2011)
Thapliyal, T., Poulos, J.L., Schmidt, J.J.: Automated lipid bilayer and ion channel measurement platform. Biosens Bioelectron 26, 2651–2654 (2011)
Watanabe, R., Soga, N., Hara, M., Noji, H.: Arrayed water-in-oil droplet bilayers for membrane transport analysis. Lab Chip 16, 3043–3048 (2016)
Fenz, S.F., Sengupta, K.: Giant vesicles as cell models. Integr. Biol 4, 982–995 (2012)
Kato, N., et al.: Effects of lipid composition and solution conditions on the mechanical properties of membrane vesicles. Membranes 5, 22–47 (2015)
Nishimura, K., et al.: Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir 28, 8426–8432 (2012)
Yang, P., Lipowsky, R., Dimova, R.: Nanoparticle formation in giant vesicles: synthesis in biomimetic compartments. Small 5, 2033–2037 (2009)
Bi, H., Ma, S., Li, Q., Han, X.: Magnetically triggered drug release from biocompatible microcapsules for potential cancer therapeutics. J Mater Chem B 4, 3269–3277 (2016)
Parigoris, E., et al.: Facile generation of giant unilamellar vesicles using polyacrylamide gels. Sci Rep 10, 1–10 (2020)
Yandrapalli, N., Petit, J., Bäumchen, O., Robinson, T.: Surfactant-free production of biomimetic giant unilamellar vesicles using PDMS-based microfluidics. Commun Chem 4, 1–10 (2021)
Lefrançois, P., Goudeau, B., Arbault, S.: Electroformation of phospholipid giant unilamellar vesicles in physiological phosphate buffer. Integr Biol 10, 429–434 (2018)
Yoshina-Ishii, C., Boxer, S.G.: Arrays of mobile tethered vesicles on supported lipid bilayers. J Am Chem Soc 125, 3696–3697 (2003)
Stamou, D., Duschl, C., Delamarche, E., Vogel, H.: Self-assembled microarrays of attoliter molecular vessels. Angew Chemie-Int Ed 42, 5580–5583 (2003)
Dusseiller, M.R., et al.: A novel crossed microfluidic device for the precise positioning of proteins and vesicles. Lab Chip 5, 1387–1392 (2005)
Al Nahas, K., et al.: A microfluidic platform for the characterisation of membrane active antimicrobials. Lab Chip 19(5), 837–844 (2019)
Deshpande, S., Caspi, Y., Meijering, A.E.C., Dekker, C.: Octanol-assisted liposome assembly on chip. Nat Commun 7, 1–9 (2016)
Paterson, D.J., Reboud, J., Wilson, R., Tassieri, M., Cooper, J.M.: Integrating microfluidic generation, handling and analysis of biomimetic giant unilamellar vesicles. Lab Chip 14, 1806–1810 (2014)
Zhu, C., Li, Q., Dong, M., Han, X.: Giant unilamellar vesicle microarrays for cell function study. Anal. Chem. 90, 14363–14367 (2018)
Schultze, J., et al.: Preparation of monodisperse giant unilamellar anchored vesicles using micropatterned hydrogel substrates. ACS Omega 4, 9393–9399 (2019)
Weinberger, A., et al.: Gel-assisted formation of giant unilamellar vesicles. Biophys J 105, 154–164 (2013)
Horger, K.S., Estes, D.J., Capone, R., Mayer, M.: Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. J Am Chem Soc 131, 1810–1819 (2009)
Han, W.B., Kwak, R., Kang, J.Y., Kim, T.S.: Generation of solvent-free 3D lipid structure arrays on high aspect ratio si microwell substrate. Adv Mater Interfaces 6, 1–9 (2019)
Han, W.B., Kang, D.H., Na, J.H., Yu, Y.G., Kim, T.S.: Enhancement of membrane protein reconstitution on 3D free-standing lipid bilayer array in a microfluidic channel. Biosens Bioelectron 141, 111404 (2019)
Kang, D., et al.: Tunable and scalable fabrication of block copolymer-based 3D polymorphic artificial cell membrane array. Nat. Commun. 13, 1261 (2022)
Acknowledgements
This work was supported by the Korean Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety) (9991006807, KMDF_PR_20200901_0134_2021_01), supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (NRF-2020R1A2C2100363), and also supported by KIST Institutional Program (2E31502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Han, W.B., Kang, DH. & Kim, T.S. 3D Artificial Cell Membranes as Versatile Platforms for Biological Applications. BioChip J 16, 215–226 (2022). https://doi.org/10.1007/s13206-022-00066-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-022-00066-z