Abstract
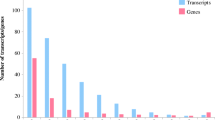
Sugarcane (Saccharum sp.) is predominantly grown in both tropics and subtropics in India, and the subtropics alone contribute more than half of sugarcane production. Sugarcane active growth period in subtropics is restricted to 8–9 months mainly due to winter’s low temperature stress prevailing during November to February every year. Being a commercial crop, tolerance to low temperature is important in sugarcane improvement programs. Development of cold tolerant sugarcane varieties require a deep knowledge on molecular mechanism naturally adapted by cold tolerant genotypes during low temperature stress. To understand gene regulation under low temperature stress, control and stressed (10 °C, 24 h) leaf samples of cold tolerant S. spontaneum IND 00-1037 collected from high altitude region in Arunachal Pradesh were used for transcriptome analysis using the Illumina NextSeq 500 platform with paired-end sequencing method. Raw reads of 5.1 GB (control) and 5.3 GB (stressed) obtained were assembled using trinity and annotated with UNIPROT, KEGG, GO, COG and SUCEST databases, and transcriptome was validated using qRT-PCR. The differential gene expression (DGE) analysis showed that 2583 genes were upregulated and 3302 genes were down-regulated upon low temperature stress. A total of 170 cold responsive transcriptional factors belonging to 30 families were differentially regulated. CBF6 (C-binding factor), a DNA binding transcriptional activation protein associated with cold acclimation and freezing tolerance was differentially upregulated. Many low temperature responsive genes involved in various metabolic pathways, viz. cold sensing through membrane fluidity, calcium and lipid signaling genes, MAP kinases, phytohormone signaling and biosynthetic genes, antioxidative enzymes, membrane and cellular stabilizing genes, genes involved in biosynthesis of polyunsaturated fatty acids, chaperones, LEA proteins, soluble sugars, osmoprotectants, lignin and pectin biosynthetic genes were also differentially upregulated. Potential cold responsive genes and transcriptional factors involved in cold tolerance mechanism in cold tolerant S. spontaneum IND 00-1037 were identified. Together, this study provides insights into the cold tolerance to low temperature stress in S. spontaneum, thus opening applications in the genetic improvement of cold stress tolerance in sugarcane.







Similar content being viewed by others
References
Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281:37636–37645
Al-Whaibi MH (2011) Plant heat-shock proteins: a mini review. J King Saud Univ Sci 23(2):139–150
Arnholdt-Schmitt B, Costa JH, de Melo DF (2006) AOX–a functional marker for efficient cell reprogramming under stress? Trends Plant Sci 11:281–287
Azevedo RA, Carvalho RF, Cia MC, Gratao PL (2011) Sugarcane under Pressure: an overview of biochemical and physiological studies of abiotic Stress. Trop Plant Biol 4:42–51
Baek KH, Skinner DZ (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci 165:1221–1227
Bhardwaj PK, Kaur J, Sobti RC, Ahuja PS, Kumar S (2011) Lipoxygenase in Caragana jubata responds to low temperature, abscisic acid, methyl jasmonate and salicylic acid. Gene 483:49–53
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Chen Y, Wang XM, Zhou L, He Y, Wang D, Qi YH, Jiang DA (2015) Rubisco activase is also a multiple responder to abiotic stresses in rice. PLoS One 10(10):e0140934
Chinnusamy V, Zhu J, Zhu JK (2006) Gene regulation during cold acclimation in plants. Physiol Plant 126:52–61
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Cho SK, Kim JE, Park JA, Eom TJ, Kim WT (2006) Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett 580:3136–3144
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signaling in plant responses to abiotic stress. J Exp Biol 217:67–75
Collins GG, Nie X, Saltveit ME (1993) Heat shock increases chilling tolerance of mung bean hypocotyl tissue. Physiol Plant 89:117–124
Conde A, Chaves MM, Geros H (2011) Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol 52:1583–1602
Degand H, Faber AM, Dauchot N, Mingeot D, Watillon B, Cutsem PV, Morsomme P (2009) Proteomic analysis of chicory root identifies proteins typically involved in cold acclimation. Proteomics 9:2903–2907
Dharshini S, Chakravarthi M, Ashwin Narayan J, Manoj VM, Naveenarani M, Kumar R, Meena M, Ram B, Appunu C (2016) De novo sequencing and transcriptome analysis of a low temperature tolerant Saccharum spontaneum clone IND 00-1037. J Biotechnol 231:280–294
Dhingra M (2015) Physiological responses and tolerance mechanisms of low temperature stress in plants. Int J Adv Res 3:637–646
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250
Du YC, Nose A, Wasano K (1999) Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant Cell Environ 22:317–324
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2015) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22:4907–4921
Ferreira SS, Hotta CT, de Carli Poelking VG, Leite DCC, Buckeridge MS, Loureiro ME, Barbosa MHP, Carneiro MS, Souza GM (2016) Co-expression network analysis reveals transcription factors associated to cell wall biosynthesis in sugarcane. Plant Mol Biol 9:15–35
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Fryer MJ, Oxborough K, Martin B, Ort DR, Baker NR (1995) Factors associated with depression of photosynthetic quantum efficiency in maize at low growth temperature. Plant Physiol 108:761–767
Gharib FA, Hegazi AZ (2010) Salicylic acid ameliorates germination, seedling growth, phytohormone and enzymes activity in bean (Phaseolus vulgaris L.) under cold stress. J Am Sci 6:675–683
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Grace SC, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos Trans R Soc Lond B Biol Sci 355:1499–1510
Gubis J, Vankova R, Cervena V, Dragunova M, Hudcovicova M, Lichtnerova H, Dokupil T, Jurekova Z (2007) Transformed tobacco plants with increased tolerance to drought. S Afr J Bot 73:505–511
Gupta V, Sharma S (2006) Plants as natural antioxidants. Indian J Nat Prod Resour 5:326–334
Hong SW, Jon JH, Kwak JM, Nam HG (1997) Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt and cold treatments in Arabidopsis thaliana. Plant Physiol 113:1203–1212
Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L (2008) Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol 67:169–181
Hu W, Yuan Q, Wang Y, Cai Deng X, Wang J, Zhou S, Chen M, Chen L, Huang C, Ma Z, Yang G, He G (2012) Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol 53:2127–2141
Huang X, Wang W, Zhang Q, Liu J (2013) A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol 162:1178–1194
Huang X, Chen MH, Yang LT, Li YR, Wu JM (2015) Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of sugarcane seedlings under cold stress. Sugar Tech 17:59–64
Karlson D, Imai R (2003) Conservation of the cold shock domain protein family in plants. Plant Physiol 131:12–15
Kolaksazov M, Laporte F, Ananieva K, Dobrev P, Herzog M, Ananiev ED (2013) Effect of chilling and freezing stresses on jasmonate content in Arabis alpina. Bulg J Agric Sci 19:15–17
Kollipara KP, Saab IN, Wych RD, Lauer MJ, Singletary GW (2002) Expression profiling of reciprocal maize hybrids divergent for cold germination and desiccation tolerance. J Plant Physiol 129:974–992
Kosova K, Prasil IT, Vitamvas P, Dobrev P, Motyka V, Flokova K, Novak O, Tureckova V, Rolcik J, Pesek B, Travnickova A, Gaudinova A, Galiba G, Janda T, Vlasakova E, Prasilova P, Vankova R (2012) Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol 169:567–576
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97:2940–2945
Lee DG, Ahsan N, Lee SH, Lee JJ, Bahk JD, Kang KY, Lee BH (2009) Chilling stress-induced proteomic changes in rice roots. J Plant Physiol 166:1–11
Lehtimaki N, Lintala M, Allahverdiyeva Y, Aro EM, Mulo P (2010) Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. J Plant Physiol 167(12):1018–1022
Li SB, Xie ZZ, Hu CG, Zhang JZ (2016) A Review of auxin response factors (ARFs) in plants. Front Plant Sci 7:47. https://doi.org/10.3389/fpls.2016.00047
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Luo HL, Weili X, Jianzhong L (2002) Study on the acceptation of Acacia mangium to low temperature stress. J South China Agricl Univ (China) 23:51–53
Lyzenga WJ, Stone SL (2012) Abiotic stress tolerance mediated by protein ubiquitination. J Exp Bot 63:599–616
Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, Xiao J, Guo X, Xu S, Niu Y, Jin J, Zhang H, Xu Li L, Wang W, Qian Q, Ge S, Chong K (2015) COLD1 confers chilling tolerance in rice. Cell 160:1209–1221
Machado DFSP, Ribeiro RV, Silveira JAG, da Filho JRM, Machado EC (2013) Rootstocks induce contrasting photosynthetic responses of orange plants to low night temperature without affecting the antioxidant metabolism. Theor Exp Plant Physiol 25:26–35
Mare C, Mazzucotelli E, Crosatti C, Francia E, Stanca AM, Cattivelli L (2004) Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 55:399–416
Masarin F, Gurpilhares DB, Baffa DC, Barbosa MH, Carvalho W, Ferraz A, Milagres AM (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol Biofuels 4:55
Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95:1321–1358
Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96(14):8271–8276
Min HJ, Jung YJ, Kang BG, Kim WT (2016) CaPUB1, a hot pepper U-box E3 ubiquitin ligase, confers enhanced cold stress tolerance and decreased drought stress tolerance in transgenic rice (Oryza sativa L.). Mol Cells 39:250–257
Miura K, Hasegawa PM (2010) Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends Cell Biol 20:223–232
Moore JP, Nguema-Ona EE, Vicre-Gibouin M, Sorensen I, Willats WGT, Driouich A, Farrant JM (2013) Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237:739–754
Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I (1992) Genetically engineered alteration in the chilling sensitivity of plants. Nature 356(6371):710
Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta Gene Regul Mech 1819:97–103
Nie GY, Robertson EJ, Fryer MJ, Leech RM, Baker NR (1995) Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant Cell Environ 18:1–12
Nogueira FT, De Rosa VE, Menossi M, Ulian EC, Arruda P (2003) RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol 132:1811–1824
O’Brien JA, Benkova E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4:451. https://doi.org/10.3389/fpls.2013.00451
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2013) Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J Exp Bot 64:445–458
Pang T, Ye CY, Xia X, Yin W (2013) De novo sequencing and transcriptome analysis of the desert shrub, Ammopiptanthus mongolicus, during cold acclimation using Illumina/Solexa. BMC Genom 14:488
Park JW, Benatti TR, Marconi T, Yu Q, Solis-Gracia N, Mora V, da Silva JA (2015) Cold responsive gene expression profiling of sugarcane and Saccharum spontaneum with functional analysis of a cold inducible saccharum homolog of NOD26-Like intrinsic protein to salt and water stress. PLoS One 10:e0125810
Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M (2008) Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep 27:1677–1686
Pearce RS (1999) Molecular analysis of acclimation to cold. Plant Growth Regul 29:47–76
Peng Y, Arora R, Li G, Wang X, Fessehaie A (2008) Rhododendron catawbiense plasma membrane intrinsic proteins are aquaporins and their overexpression compromises constitutive freezing tolerance and cold acclimation ability of transgenic Arabidopsis plants. Plant, Cell Environ 3:1275–1289
Ram B, Sreenivasan TV, Sahi BK, Singh N (2001) Introgression of low temperature tolerance and red rot resistance from Erianthus in sugarcane. Euphytica 122:145–153
Reddy VS, Reddy AS (2004) Proteomics of calcium-signaling components in plants. Phytochemistry 65:1745–1776
Renaut J, Lutts S, Hoffmann L, Hausman JF (2004) Responses of poplar to chilling temperatures: proteomic and physiological aspects. Plant Biol 7:81–90
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571. https://doi.org/10.3389/fpls.2016.00571
Sales CRG, Ribeiro RV, Machado DFSP, Machado RS, Dovis VL, Lagôa AMMA (2012) Gas exchange and carbohydrate balance in sugarcane plants under root stressful conditions. Bragantia 71:319–327
Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638
Sato Y (2001) Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J Exp Bot 52:145–151
Shi HT, Li RJ, Cai W, Liu W, Wang CL, Lu YT (2012a) Increasing nitric oxide content in Arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol 53:344–357
Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012b) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and Type-A ARR genes in Arabidopsis. Plant Cell 24:2578–2595
Shi Y, Ding Y, Yang S (2014) Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol 56(1):7–15
Solanke AU, Sharma AK (2008) Signal transduction during cold stress in plants. Physiol Mol Biol Plants 14:69–79
Solecka D, Zebrowski J, Kacperska A (2008) Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann Bot 101:521–530
Solomon S (2016) Sugarcane production and development of sugar industry in India. Sugar Tech 18(6):588–602
Song Y, Liu L, Wei Y, Li G, Yue X, An L (2017) Metabolite profiling of adh1 mutant response to cold stress in Arabidopsis. Front Plant Sci 7:2072
Strauss G, Hauser H (1986) Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc Natl Acad Sci USA 83:2422–2426
Sze H, Liang F, Hwang I, Curran AC, Harper JF (2000) Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Biol 51:433–462
Szekely G, Abraham E, Cseplo A, Rigo G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jasik J, Schmelzer E, Koncz C (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Tarkowski LP, Van den Ende W (2015) Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci 6:203. https://doi.org/10.3389/fpls.2015.00203
Thalhammer A, Hincha DK (2014) A mechanistic model of COR15 protein function in plant freezing tolerance: integration of structural and functional characteristics. Plant Signal Behav 9:e977722
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Biol 50(1):571–599
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteom 71:391–411
Tiwari M, Sharma D, Singh M, Tripathi RD, Trivedi PK (2014) Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci Rep 4:3964 (PMCID: PMC3912489 )
Urao T, Miyata S, Yamaguchi-Shinozaki K, Shinozaki K (2000) Possible His to Asp phosphorelay signaling in an Arabidopsis two component system. FEBS Lett 478:227–232
Van Buskirk HA, Thomashow MF (2006) Arabidopsis transcription factors regulating cold acclimation. Physiol Plant 126:72–80
Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Biol 48(1):703–734
Verslues PE, Zhu JK (2005) Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem Soc Trans 33:375–379
Virdi AS, Singh S, Singh P (2015) Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front Plant Sci 6:809. https://doi.org/10.3389/fpls.2015.00809
Viswanathan C, Zhu J (2002) Molecular genetic analysis of cold-regulated gene transcription. Phil Trans R Soc Lond B. 357:877–886
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41:195–211
Wang CY, Adams DO (1982) Chilling-induced ethylene production in cucumbers (Cucumis sativus L.). Plant Physiol 69:424–427
Wang B, Guo G, Wang C, Lin Y, Wang X, Zhao M, Guo Y, He M, Zhang Y, Pan L (2010) Survey of the transcriptome of Aspergillus oryzae via massively parallel mRNA sequencing. Nucleic Acids Res 38(15):5075–5087
Wang XH, Shu C, Li HY, Hu XQ, Wang YX (2014) Effects of 0.01% brassinolide solution application on yield of rice and its resistance to autumn low-temperature damage. Acta Agric Jiangxi 26:36–38
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:1–15
Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell Environ 33:510–525
Wright M (1974) The effect of chilling on ethylene production, membrane permeability and water loss of leaves of Phaseolus vulgaris. Planta 120:63–69
Wu G, Wilen RW, Robertson AJ, Gusta LV (1999) Isolation, chromosomal localization, and differential expression of mitochondrial manganese superoxide dismutase and chloroplastic copper/zinc superoxide dismutase genes in wheat. Plant Physiol 120:513–520
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:165–183
Yan SP (2005) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteom 5:484–496
Yan J, He C, Wang J, Mao Z, Holaday SA, Allen RD, Zhang H (2004) Overexpression of the Arabidopsis 14-3-3 protein GF14 lambda in cotton leads to a “stay-green” phenotype and improves stress tolerance under moderate drought conditions. Plant Cell Physiol 45:1007–1014
Yang T, Shad Ali G, Yang L, Du L, Reddy SN, Poovaiah BW (2010) Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal Behav 5:991–994
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot 63:2541–2556
Yang YW, Chen HC, Jen WF, Liu LY, Chang MC (2015) Comparative transcriptome analysis of shoots and roots of TNG67 and TCN1 rice seedlings under cold stress and following subsequent recovery: insights into metabolic pathways, phytohormones, and transcription factors. PLoS One 10:0131391
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Zhang Z, Huang R (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 73:241–249
Zhang B, Chen K, Bowen J, Allan A, Espley R, Karunairetnam S, Ferguson I (2006) Differential expression within the LOX gene family in ripening kiwifruit. J Exp Bot 57:3825–3836
Zhang G, Guo G, Hu X, Zhang Y, Li Q, Li R, Zhuang R, Lu Z, He Z, Fang X, Chen L, Tian W, Tao Y, Kristiansen K, Zhang X, Li S, Yang H, Wang J, Wang J (2010) Deep RNA sequencing at single base-pair resolution reveals high complexity of the rice transcriptome. Genome Res 20(5):646–654
Zhang BQ, Yang LT, Li YR (2014) Comparison of physiological and biochemical characteristics related to cold resistance in sugarcane under field conditions. Sugar Tech 17:496–505
Zhao C et al (2017) MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev Cell 43:618–629
Zhu JJ, Li YR, Liao JX (2013) Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. leaves. Plant Physiol Biochem 73:427–433
Acknowledgements
The authors thank ICAR-Sugarcane Breeding Institute, Coimbatore for providing the necessary infrastructure. We would like to thank Dr. G. Hemaprabha, Head, Division of Crop Improvement and Dr. N. Subramonian, Emeritus Scientist, ICAR-SBI for their critical comments on the content. Thanks to Mr. K. Selvamuthu for his technical assistance to carry out the work.
Funding
The authors thank the Science and Engineering Research Board (SERB), Department of Science and Technology for financial support (Grant No.SB/YS/LS-165/2013). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
DS and AC designed and performed the experiments. DS, CM, ANJ, MVM, NM CM, RK and MM wrote the manuscript. VD did the MapMan analysis of the data. GN did the artwork for figures. AC and BR revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Selvarajan, D., Mohan, C., Dhandapani, V. et al. Differential gene expression profiling through transcriptome approach of Saccharum spontaneum L. under low temperature stress reveals genes potentially involved in cold acclimation. 3 Biotech 8, 195 (2018). https://doi.org/10.1007/s13205-018-1194-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1194-2