Abstract
Extracellular Polysaccharides (EPS) from both prokaryotes and eukaryotes have a great deal of research interest as they protect the producer from different stresses including antibiotics, ionic stress, desiccation and assist in bio-film formation, pathogenesis, adhesion, etc. In this study haloalkaliphilic Bacillus sp., known to cope with osmophilic stress, was selected and screened for EPS production. The EPS were isolated, partially purified and chemical characteristics were documented using liquid FT-IR followed by assessment of heavy metal biosorption (lead, copper and cadmium) using Atomic Absorption Spectroscopy (AAS). The EPS extracted from three isolates B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 showed maximum biosorption of Lead followed by Copper and Cadmium. Of the tested isolates, the EPS from isolate B. cereus NSPA8 showed maximum (90 %) biosorption of the lead.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
EPS are polymeric substances excreted by the microorganisms when growing in environment of abundance or after establishing in a suppressing one and play a major role in the formation of bio-films to grow on the substrates in environments where normally others cannot colonise (Sutherland 2001). In some species, EPS play an essential role in imparting antibiotic resistance to the organism by denying permeability to the antibiotics (Costerton et al. 1987).
Depending on the genera these EPS are made of different carbohydrates, proteins and their derivatives as hetero as well as homo-polymers, which make them potential contender for multiple field applications. EPS are being actively employed in food, bioremediation and pharmaceutical sectors owing to their gelling nature which allows them to be used as preservative, viscosifying agent, flavouring agent and form super-absorbing gels, biosorption agents etc., (Sutherland 1998; Challouf et al. 2011). It is well established that these EPS have large surface area of interaction particularly with cations (metals), which comes handy during surviving in diverse contaminated areas and the same is applicable in remediation of heavy metal contamination (Pal and Paul 2008).
In this study, a moderately haloalkaliphilic bacteria of the genus Bacillus was studied from artificial hyper saline habitats i.e., solar salterns, which are wide spread in distribution and primarily present in the tropical and sub-tropical regions constructed by humans near coastal regions for the purpose of edible salt production. As the current knowledge regarding potential applications of microbes from artificial hyper saline environments and their applicability in metal detoxification is very limited, this study was focused to evaluate the metal biosorption ability of EPS extracted from three different types of haloalkaliphilicbacteria.
Materials and methods
Isolation, selection and characterisation of Bacillus sp., from solar salterns
For the isolation of the Bacillus sp., 1.0 g of soil sample was collected from solar salterns by employing standard method of soil sampling (Carter 1993) and was inoculated into 100 ml of the modified nutrient medium with 7 % NaCl and the final pH was adjusted to 8.2. After inoculation, flasks were incubated on orbital shaker at 130 rpm/min with regular monitoring of the turbidity of the media at 37 °C. After 48–72 h of growth, loop full of culture was spread plated/pour plated on the nutrient agar (Agar 1.5 % w/v) plate and incubated at 37 °C for 5 days. Based on the colony characteristics such as form, elevation and margin various discrete and distinct colonies were selected and purified. The selected isolates were screened for standard biochemical reactions to establish preliminary identity of the isolates (Cappuccino et al. 2005). Molecular characterization was carried out according to the protocols of Sambrook et al. (1989), Saiki et al. (1988), Weisburg et al. (1991) and Higgins et al. (1992) for genomic DNA isolation, PCR amplification, Amplicon sequencing and phylogenetic relationship analysis, respectively.
Screening for extracellular polysaccharides (EPS) production
EPS production was screened by visual and by microscopic observation. The colonies were picked from pure culture plates of three potential isolates and spread plated using a disposable L-rod and incubated at 37 °C for 48 h. Mucoidness of colonies was determined by visual appearance and the ropiness of colonies in liquid broth was determined by testing with a sterile inoculation needle. Further EPS production was also confirmed by scanning electron microscopy according to Bulla (1970) and Kaláb et al. (2008) where the isolate’s EPS layers were picked up and transferred to a particle free graphite cover slip and dried at 35 °C and processed for SEM imaging.
Production and extraction of extracellular polysaccharides
All the three isolates were inoculated into EPS production medium containing Glucose 10.0 g/L, Yeast extract 3.0 g/L, Malt extract 3.0 g/L, Peptone 5.0 g/L, MgSO4·7H2O 1.0 g/L, KH2PO40.3 g/L, vitamin B1 0.001 g/Land pH 7.0 (Banerjee et al. 2009) and incubated in rotatory shaking incubator at 180 rpm for 96 h at 40 °C. After 96 h of incubation the cultures were treated with 10 psi steam for 20 min to loosen the attached polymer, centrifuged at 8000 rpm and supernatant was collected. To the supernatant, chilled Iso-proponal was added in equal volume. This mixture was agitated for few minutes and left overnight at 4 °C (Brown and Lester 1980; Donota et al. 2012). The precipitated EPS were separated by centrifugation at 7000 rpm for 20 min. The supernatant was dried in an oven at 60 °C until it reaches constant weight. The precipitate was used for protein analysis and dried supernatant was used for total carbohydrate and reducing sugars estimation.
Dry weight of the extracellular polysaccharides
Extracted EPS were dried in a pre weighed density bottles at 60 °C till it attains constant weight. Dry weight of polymer was calculated in relation to the volume of supernatant used for extraction (Ohno et al. 2001).
A weight of empty density bottle, B weight of bottle with dry polymer
Characterisation of extracellular polysaccharides
Characterisation of physical properties of EPS
The physical properties of EPS such as colour and texture were analysed by macroscopic and Electron microscopic observation. The dried EPS layer was placed on the particle free carbon tape and attached to the metallic studs and placed in the Gold sputtering chamber to coat the particles to create electron dense and sparse regions. The difference in the gold coated particles gives dark and brighter regions creating an electron image (Sutton et al. 1994).
Chemical composition of extracellular polysaccharides
The extracted EPS were subjected to total carbohydrate and reducing sugar estimation as described by Dubois et al. (1956), where 2.0 ml of EPS solution was taken into a test tube and 0.05 of 80 % phenol is added followed by rapid addition of 5.0 ml concentrated sulphuric acid. The tubes were allowed to stand for 10 min then, shaken well and placed in water bath at 30 °C for 20 min. The absorbance was measured at 490 nm and calculated against a standard of glucose treated similarly.
The reducing sugars were estimated following DNS method, where 1.0 ml of EPS was mixed with 3.0 ml of DNS reagent and kept in a boiling water bath for 5.0 min and allowed to cool and absorbance was measured at 540 nm, concentration was calculated using standard curve.
The EPS were subjected to protein estimation as per Lowry et al. (1951), where to 1.0 ml of EPS solution 4.5 ml of alkaline copper sulphate reagent is added and incubated at room temperature for 10 min. Then 0.5 ml of Folin-Phenol reagent is added and incubated for further 30 min and absorbance was measured at 660 nm, protein concentration was calculated against a standard curve.
Structural elucidation of the extracellular polysaccharides
Structural elucidation of the EPS was done using Fourier Transform Infrared Spectroscopy (FT-IR, from Perkin Elmer, Germany). For FT-IR analysis, 2.0 mg of extracted EPS was grounded with 200 mg of KBr and then pressed in a mould. The absorption spectrum was recorded in the frequency range of 4000–400 cm−1 to analyse different functional groups present in the EPS. Thus, obtained pellets were analysed for different functional groups to asses’ structural characters (Lijour et al. 1994; Verhoef et al. 2005; Denkhaus et al. 2007).
Metal biosorption by extracellular polysaccharides
The metal biosorption potential of the EPS against heavy metals Lead, Copper and Cadmium was studied by adding the whole EPS at 1.0 mg/100 ml of the test metal solution having 1000 ppm of respective metal at 37 °C under constant stirring of 250 rpm for 24 h. After incubation, the EPS were separated by centrifugation at 10,000 rpm and the metal biosorption was measured using Atomic Absorption Spectroscopy (AAS) (Bass et al. 2001). The metal biosorption potential is deducted by calculating as follows (Volesky and Holan 1995). Metal removal (q), from the solution was expressed as mg metal removed/g dry weight−1, which was calculated using the following formula
where V is the sample volume (l), C i and C f are the initial and final metal concentrations (mg/l), respectively, m is the amount (g) of dry biomass.
Results
Extracellular Polysaccharides production and applications
Isolation, selection and characterisation of Bacillus sp., from solar salterns
A total of 14 bacterial isolates were initially isolated from solar salterns on modified nutrient agar medium and the isolates were selected on the basis of cultural characteristics such as colony size, colour, form, margin and elevation. Based on the Bergey’s manual of systemic bacteriology, those fitting the description of Bacillus sp were selected for molecular characterisation (Bergey et al. 1939). The selected isolates were taxonomically classified using phylogenetic analysis. The amplified 16S rDNA gene using polymerase chain reaction resulted in a single discrete band of a 1.5 kb size in agarose gel. This amplified PCR product was BLAST searched against NCBI Genbank and RDP (Ribosomal Database Project) database 11.0. A distance matrix was constructed based on nucleotide sequence homology using kimura-2 parameter and phylogenetic trees were made using neighbour joining method. Based on nucleotide homology and phylogenetic analysis the isolates NSPA5, NSPA8 and NSPA13 showed highest similarity (99.0 %) with Bacillus licheniformis (Genbank Accession no AB301011) and nearest homolog was found to be Bacillus sp. (Genbank FR823409), Bacillus cereus st.GUFBSS253-84 (Genbank. JN315893) 99 %, respectively, and nearest homolog was found to be Bacillussp.BP9_4A (Genbank JN644555) and Bacillus subtilis st.HS-116 (Genbank JQ062996) 99 % and nearest homolog was found to be Bacillus subtilis st.69 (Genbank JN582031), respectively. The sequences were submitted to Genbank with Accession No: JQ922113, KC686834 and KC686835 for the sequences of NSPA5, NSPA8 and NSPA13respectively (Fig. 1).
Screening for extracellular polysaccharide (EPS) production
The isolates B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 showed mucoidal growth during their isolation and purification. The bacterial cells were clearly observed as encapsulated by the EPS through capsule staining and gram’s staining observations. Further the SEM images clearly established the presence of extracellular polymer attached to the bacterial cells, and the cells grew as a Bio-film. Images showing the Bio-film formation are presented below (Figs. 2, 3).
Dry weight of the extracellular polysaccharides
The EPS total dry weight of the isolates B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 measured up to around 22.25, 21.2 and 19.3 mg/10 ml, respectively. The amount of EPS produced varied even though the growth medium and condition were identical.
Characterisation of extracellular polysaccharides
Characterisation of physical properties of EPS
The EPS of the isolates was granular powder in case of B. licheniformis NSPA5 and B. subtilis NSPA13 but that of B. cereus NSPA8 was finely amorphous in nature from naked eye examination. Similar characteristics were established by SEM image analysis of the dried powdered EPS and mucoidal layers of the isolates. SEM imaging elucidates Mucoidal layers as intact relatively long polymeric in nature; while the precipitated EPS is highly fragmented when compared with mucoidal layers. The isolate B. cereus NSPA8 has very thin polymeric chains than the rest of the isolates i.e., B. licheniformis NSPA5 and B. subtilis NSPA13. It is clearly evident that alcohol treatment almost completely denatured the EPS of all the isolates apparent from the SEM images as shown below (Fig. 4) when compared with the untreated ones (Fig. 5).
Chemical composition of EPS
The total carbohydrate content in the Extracellular polysaccharide after extraction by precipitation was found to be 2.8, 4.4 and 3 mg/100 mg of the total EPS produced by B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13, respectively. Total carbohydrate determination shows isolate B. cereus NSPA8 has produced maximum and B. licheniformis NSPA5 the least amount of carbohydrates among the three tested isolates. The same were found to be comprised of reducing sugars which were found to be around 2, 2 and 1.8 mg/100 mg of EPS of B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13, respectively. The isolates B. licheniformis NSPA5 and B. cereus NSPA8 contained similar amount of reducing sugars in their EPS. The total protein content in the EPS was 4.3, 2.5 and 3.5 mg/100 mg of EPS from B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13, respectively; isolate B. licheniformis NSPA5 produced maximum content in its EPS. The results are presented in Fig. 6.
Structural elucidation of the EPS
IR spectra of EPS from isolates are presented in Fig. 7. The spectra of three EPS are similar and indicate the presence of the same functional groups mentioned in Table 1. Several intense characteristic bands can be attributed to protein and polysaccharide functional groups. These different functional groups observed agree with results of (Guibaud 2003, 2005) and are in accordance with the EPS biochemical composition.
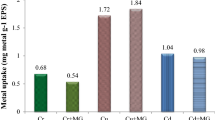
Metal biosorption by EPS of B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 atomic absorption spectroscopy (AAS)
After analysing the treated samples in AAS, the EPS of isolate B. cereus NSPA8showed maximum biosorption of the tested metals. The results show all the three isolates were able to adsorb lead at a concentration of 1000 ppm. The metals which expressed lesser inhibition effect in the metal tolerance assay in this case copper and cadmium were the least adsorbed ones; the metal concentration in the bacterial treated medium is reduced to 210.45, 140.2 and 154.65 ppm by the EPS of B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13, respectively, in the case of Lead. Copper biosorption was somewhat different as the EPS showed varied biosorption when compared with other two metals; all the three EPS’s showed very distinct abilities as compared with Lead and Cadmium. The EPS of B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 reduced the metal concentration to 985.42, 840.5 and 920.48 ppm, respectively, from the original 1000 ppm concentration. In case of copper biosorption all the EPS’s limited themselves to reducing the initial metal concentration to around 942.85, 945.03 and 928.28 ppm by the EPS of B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13, respectively, showing uniformity in the copper biosorption ability unlike with Lead and Cadmium.
The biosorption studies of the selected metal showed that the metal’s toxicity plays crucial role in the biosorption ability, the EPS of B. cereus NSPA8 showed maximum biosorption of all the three tested metals except copper. Lead was the maximum adsorbed one at almost 90 %. The EPS of B. licheniformis NSPA5 and B. subtilis NSPA13 also showed maximum biosorption of lead but still less when compared with B. cereus NSPA8. The other two metals cadmium and copper were too adsorbed but, negligible when compared with lead. The metal biosorption efficiency of the EPS of the isolates is depicted in the Fig. 8 below as determined from AAS.
Discussion
In recent years, exopolysaccharides (EPS) produced by bacteria have been employed in diverse fields ranging from food to pharma. So, in this study the EPS produced by the three isolates B. licheniformis NSPA5, B. cereus NSPA8 and B. subtilis NSPA13 were extracted and its characteristics and metal biosorption were studied. The chemical and dry weight analysis revealed the EPS from the isolates has both carbohydrate and protein components and the quantity produced were more or less in agreement with previous reports (Maria et al. 1996; Shih et al. 2010). The same was contradicted by reports from Mitsuda et al. (1981) and Shih et al. (2010) where EPS were majorly composed of carbohydrates and their derivatives. FT-IR analysis showed the presence of active carboxylic groups and the results were in harmony with reports other workers like Suh et al. (1993) and Ganesh et al. (2004) where Haloalkaliphilic Bacillus sp. was studied. The EPS when screened for heavy metal biosorption showed variation in results to that of previous reports (James 1986; Chen et al. 2002; Salehizadeh and Shojaosadati 2003; Yilmaz 2003) when Bacillus sp, was studied. The major difference being EPS in this study has significant biosorption of Lead only but above reports indicates it was the least biosorbed. In case of Copper and Cadmium which was higher in their reports, our study showed only minimal biosorption. All the isolate’s EPS biosorbed the tested metals Lead, Cadmium and Copper, but Lead was the most sorbed by all the isolates as determined by AAS at a concentration of 1000 ppm, but previous studies were with lower concentrations at around 100 ppm only. Thus, EPS from haloalkaliphilic Bacillus sp. have potential applications in treatment of metal contaminated waters specially lead contamination as proposed by Ha et al. (1991) (Loaëc et al. 1997; Beyenal and Lewandowski 2004; Pal and Paul 2008).
References
Abu Sayem S, Manzo E, Ciavatta L, Tramice A, Cordone A, Zanfardino A (2011) Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb Cell Fact 10:74
Ashtaputre AA, Shah AK (1994) Studies on a viscous, gel-forming exopolysaccharide from Sphingomonas paucimobilis GS1. Appl Environ Microbiol 61:1159–1162
Banerjee D, Jana M, Mahapatra S (2009) Production of exopolysaccharide by endophytic Stemphylium sp. Micol Aplic Int 21(2):57–62
Bass DA, Hickok D, Quig D, Urek K (2001) Trace element analysis in hair: factor determing accuracy, precision and reliability. Altern Med Rev 6(5):472–481
Bauer AW, Perry DM, Kirby WMM (1959) Single disc antibiotic sensitivity testing of Staphylococci. A.M.A. Arch Intern Med 104:208–216
Bergey DH, Breed RS, Murray EGD, Hitchens AP (1939) Bergey’s manual of determinative bacteriology, 5th edn. The Williams & Wilkins Company, Baltimore
Beyenal H, Lewandowski Z (2004) Dynamics of lead immobilization in sulfate reducing biofilms. Water Res 38:2726–2736
Brady CL, Venter SN, Cleenwerck I, Engelbeen K, Vancanneyt M, Swings J, Coutinho TA (2009) Pantoea vagans sp. nov., Pantoea eucalypti sp. nov., Pantoea deleyi sp. nov. and Pantoea anthophila sp. nov. Int J Syst Evol Micr 59:2339–2345
Britten M, Morin A (1995) Functional characterization of the exopolysaccharide from Enterobacter agglomerans grown on low-grade maple. Food Sci Technol 28:264–271
Brown MJ, Lester JN (1980) Comparison of bacterial extracellular polymer extraction methods. Appl Environ Microbiol 40(2):179–185
Bulla LA (1970) SEM of microorganisms. Micro-Views 2:1–3
Cappuccino JG, Sherman N (2005) Microbiology: a laboratory manual. Pearson Education Pte. Ltd, Singapore
Carter MR (1993) Soil sampling and methods of analysis. CRC Press
Challouf Rafika, Trabelsi Lamia, Dhieb Ben (2011) Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospiraplatensis. Brazil Arch Biol Technol 54(4):831–838
Chen JP, Lie D, Wang L, Wu S, Zhang B (2002) Dried waste activated sludge as biosorbents for metal removal: adsorptive characterization and prevention of organic leaching. J Chem Technol Biotechnol 77:622–657
Comte S, Guibaud G, Baudu M (2006) Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties Part I. comparison of the efficiency of eight EPS extraction methods. Enz Microbial Technol 38:237–245
Corsaro M, De Castro M, Evidente C, Lanzetta A, Molinaro R, Parilli A, Sparapano L (1998) Phytotoxic extracellular polysaccharide fractions from Cryphonectriaparasitica (Murr) Barr strains. Carbohyd Polym 37:167–172
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptidebiosurfactants derived from a marine Bacillus circulans. J Appl Microbiol 04:675–684
De Belder AN (1993) Dextran in industrial gums. Accademic Press, San Diego, pp 399–425
Denkhaus E, Meisen S, Telgheder U, Wingender J (2007) Chemical and physical methods for characterisation of biofilms. Microchim Acta 158:1–27
Donota F, Fontanaa A, Baccoua JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87:951–962
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fenice M, Gallo AM, Juarez-Jimenez B, Gonzalez-Lopez J (2007) Screening for extracellular enzyme activity by bacteria isolated from samples collected in the Tyrrhenian Sea. Ann Microbiol 57:93–100
Fialho AM, Moreira LM, Granja A, Popescu AO, Hoffmann K, Sá-Correia I (2008) Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biot 79:889–900
Flemming H, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Ganesh KC, Han-Seung J, Jang-Won C, Yoon-Moo K, Chung-Soon C (2004) Purification and characterization of an extracellular polysaccharide from haloalkaliphilic Bacillus sp. I-450. Enzyme Microb Technol 34:673–681
Grassi M, Colombo I, Lapasin R (2000) Drug release from an ensemble of swellable crosslinked polymer particles. J Control Release 68:97–113
Guibaud G, Tixier N, Bouju A, Baudu M (2003) Relation between extracellular polymers composition and its ability to complex Cd, Cu and Pb. Chemosphere 52:1701–1710
Guibaud G, Comte S, Bordas F, Dupuy S, Baudu M (2005) Comparison of the complexation potential of extracellular polymeric substances (EPS) extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel. Chemosphere 59:629–638
Ha YW, Stack R, Hespell RB, Gordon SH, Bothast RJ (1991) Some chemical and physical properties of extracellular polysaccharides produced by Butyrivibriofibrisolvensstrains. Appl Environ Microbiol 57:2016–2020
Higgins DG, Bleasby AJ, Fuchs R (1992) CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci 8(2):189–191
Høiby N, Ciofu O, Bjarnsholt T (2010) Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 5:1663–1674
Iwabuchi N, Sunairi M, Urai M, Itoh C, Anzai H, Nakajima M, Harayama S (2002) Extracellular polysaccharides of Rhodococcusrhodochrous S-2 stimulate the degradation of aromatic components in crude oil by indigenous marine bacteria. Appl Environ Microbiol 68(5):2337–2343
James KC (1986) Drugs and the pharmaceutical sciences: solubility and related properties, vol 28. Marcel Dekker, New York, pp 91–125
Kaláb M, Yang AF, Denise C (2008) Conventional Scanning Electron Microscopy of Bacteria. Infocus, Proce R Microscop Soc 6:42–61
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides: a perception. J Basic Microbiol 47:103–117
Lee IY, Seo WT, Kim GJ, Kim MK, Ahn SG, Kwon GS, Park YH (1997) Optimization of fermentation conditions for production of exopolysaccharide by Bacillus polymyxa. Bioprocess Eng 16:71–75
Lee HK, Chun J, Moon EY, Ko SH, Lee DS, Lee HS, Bae KS (2001) Hahellachejuensis gen. nov., sp. nov., an extracellular-polysaccharide-producing marine bacterium. Int J Syst Evol Microbiol 51:661–666
Lijour Y, Gentric E, Deslandes EGJ (1994) Estimation of the sulfate content of hydrothermal vent bacterial polysaccharides by Fourier transform infrared spectroscopy. Analyt Biochem. 220:244–248
Loaëc M, Olier R, Guezennec J (1997) Uptake of lead, cadmium and zinc by a novel bacterial exopolysaccharide. Water Res 31:1171–1179
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Mah T, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Maria CM, Licia L, Roberta I, Enrico E, Agata G, Barbara N (1996) Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 62(9):3265–3269
Martínez-Checa F, Toledo F, Vilchez R, Quesada E, Calvo C (2002) Yield production, chemical composition, and functional properties of emulsifier H28 synthesized by Halomonaseurihalina strain H-28 in media containing various hydrocarbons. Appl Microbiol Biot 58:358–363
Mitsuda SN, Miyata N, Hirota T, Kikuchi T (1981) High-viscositypolysaccharide produced by Bacillus polymyxa. Hakkokogaku 59:303–309
Moslemy P, Guiot SR, Neufeld RJ (2004) Activated sludge encapsulation in gellan gum microbeads for gasoline biodegradation. Bioprocess Biosyst Eng 26:197–204
Munn C (2011) Marine Microbiology, ecology and applications. Garland Science, New York
Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T (2001) Antitumor β-glucan from the cultured fruit body of Agaricusblazei. Biol Pharm Bull 24:820–828
Pal A, Paul AK (2008) Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J. Microbiol. 48:49–64
Poli A (2010) Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar Drugs 8:1779–1802
Pollock TJ, Michael A (2007) Sphingan group of exopolysaccharides (EPS). Biopolymers. In A. Steinbuechel. Wiley-VCH, Weinheim, pp 239–258
Saiki RK, Gelfand DH, Stoffel S, Scharf ST, Higuchi R, Horn GT, Ehrlich HA (1988) Primer-directed enzymatic amplification of DNA. Science 239:487–491
Salehizadeh H, Shojaosadati SA (2003) Removal of metalions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37:4231–4235
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold spring harbor laboratory press, New York
Satpute SK, Banat IM, Dhakephalkar PK, Banpurkar AG (2010) Chopade, biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol 28:436–450
Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M (2002) Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phomaherbarum CCFEE 5080. Research Microbiol 153:585–592
Shih IL, Chen LD, Wu JY (2010) Levan production using Bacillus subtilis natto cells immobilized on alginate. Carbohydr Polym 82(1):111–117
Suh HH, Lee MH, Kim HS, Park CS, Yoon BD (1993) Bioflocculant production from Bacillus sp. A56. Korean J Appl Microbiol Biotechnol 21:486–493
Sutherland IW (1998) Novel and established applications of microbial Polysaccharides. Trends Biotechnol 16:41–46
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:4–9
Sutton NA, Hughes N, Handley PS (1994) A comparison of conventional SEM techniques, low temperature SEM and the electro scan wet scanning electron microscope to study the structure of a biofilm of Streptococcus cristaCR3. J Appl Bacteriol 76(5):448–454
Thenmozhi R, Nithyanand P, Rathna J, Pandian SK (2009) Anti-biofilm activity of coral associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol 57:284–294
Verhoef Rene et al (2005) Sugar composition and FT-IR analysis of exopolysaccharides produced by microbial isolates from paper mill slime deposits. Biotechnol and bioengin. 91(1):91–105
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Wang Y, McNeil B (1995) Production of the fungal exopolysaccharide scleroglucan by cultivation of Sclerotium glucanicum in airlift reactor with an external loop. J Chem Tech Biotechnol 63:215–222
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriology 173(2):697–703
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances. In Microbial Extracellular Polymeric Substances: characterization, structure and function. Edited by Wingender J, Neu TR, Flemming HC. Springer-Verlag, Berlin, pp 1–19
Wood TK, Hong SH, Ma Q (2011) Engineering biofilm formation and dispersal. Trends Biotechnol 29:87–94
Yilmaz IE (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res Microbiol 154:409–415
Acknowledgments
Authors are thankful to Sri Venkateswara University, Tirupati, India for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest regarding the publication of this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shameer, S. Biosorption of lead, copper and cadmium using the extracellular polysaccharides (EPS) of Bacillus sp., from solar salterns. 3 Biotech 6, 194 (2016). https://doi.org/10.1007/s13205-016-0498-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-016-0498-3