Abstract
Biosurfactants have gained a renewed interest in the recent years for their commercial application in diverse research areas. Recent evidences suggest that the antimicrobial activities exhibited by biosurfactants make them promising molecules for the application in the field of therapeutics. Marine microbes are well known for their unique metabolic and functional properties; however, few reports are available till date regarding their biosurfactant production and antimicrobial potential. In an ongoing survey for bioactive microbial metabolites from microbes isolated from diverse ecological niches, a marine Staphylococcus saprophyticus SBPS 15 isolated from the petroleum hydrocarbon contaminated coastal site, Puducherry, India, was identified as a promising biosurfactant producer based on multiple screening methods. This bacterium exhibited growth-dependent biosurfactant production and the recorded yield was 1.345 ± 0.056 g/L (on dry weight basis). The biosurfactant was purified and chemically characterized as a glycolipid with a molecular mass of 606.7 Da, based on TLC, biochemical estimation methods, FT-IR spectrum and MALDI-TOF–MS analysis. Further, the estimated molecular mass was different from the earlier reports on biosurfactants. This new glycolipid biosurfactant exhibited a board range of pH and temperature stability. Furthermore, it revealed a promising antimicrobial activity against many tested human pathogenic bacterial and fungal clinical isolates. Based on these observations, the isolated biosurfactant from the marine S. saprophyticus revealed board physicochemical stabilities and possess excellent antimicrobial activities which proves its significance for possible use in various therapeutic and biomedical applications. To the best of our knowledge, this is the first report of a biosurfactant from the bacterium, S. saprophyticus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosurfactants possess both hydrophilic and hydrophobic moieties that tend to interact with the phase boundary between two distinct phases in a heterogeneous system to solubilize them (Ron and Rosenberg 2002). Biosurfactants has several advantages over their synthetic counterparts such as higher biodegradability; lower toxicity; good biocompatibility; stable at different physico-chemical conditions; synthesis under user-friendly conditions, e.g., low temperatures and pressures (Zhang et al. 2004; Ruggeri et al. 2009). They exhibit diverse functional properties such as emulsification, wetting, foaming, cleansing, phase separation, surface activity and reduction in viscosity of crude oil, which makes them amenable for the application in diverse niche areas such as agriculture, pharmaceuticals, cosmetics, food industries, oil recovery and environmental remediation (Mulligan 2005; Campos et al. 2013; Sachdev and Cameotra 2013; Gudina et al. 2016).
In addition to these, recent studies evidenced that biosurfactants exhibited anti-bacterial, anti-fungal and anti-viral activities which makes them potential sources for biomedical applications (Singh and Cameotra 2004). Many of the known biosurfactants having bioactive potential have been reported from terrestrial habitats, the well known biosurfactants are surfactin, fengycin, bacillomycins and iturin produced by Bacillus subtilis (Ahimou et al. 2000), sophorolipids (Cavalero and Cooper 2003), rhamnolipids from Pseudomonas aeruginosa (Benincasa et al. 2004) and mannosylerythritol lipids from Candida antarctica (Arutchelvi et al. 2008). Despite the fact that marine environment forms >70 % of the Earth’s biosphere comprising of diverse group of microorganisms with unique metabolic, structural and functional properties (Fenical 1993; Proksch et al. 2003); they were less studied till date.
The first antimicrobial marine biosurfactant was reported from Bacillus circulans isolated from the Andaman and Nicobar Islands, India, which exhibited antimicrobial potential against several multidrug resistant human pathogens with non-hemolytic property (Das et al. 2008). Thereafter, some researchers explored the antimicrobial activities of biosurfactants from marine microbes (Khopade et al. 2012; Dusane et al. 2011; Kiran et al. 2010). However, when compared to biosurfactants from terrestrial isolates, few marine biosurfactants have been explored for its antimicrobial potential, hence warrants this investigation.
Staphylococcus is a well known genus for its human and animal infections, but some species have been recognized as common commensals. Recent studies have reported that some secondary metabolites produced by Staphylococcus species isolated from natural environments exhibited biotechnological and biomedical significance (Popowicz et al. 2006). Staphylococcus saprophyticus isolated from the seawater in Jiangsu, China showed appreciable production of lipase having excellent organic solvent tolerance (Fang et al. 2006). Moreover, previous studies showed the possibilities of biosurfactant production in the genus Staphylococcus species (Eddouaouda et al. 2012). To the best of our knowledge, no studies have been reported on Staphylococcus saprophyticus for biosurfactant production. Hence, the present study was undertaken with regard to the isolation of potential biosurfactant strains using multiple screening methods, followed by the production and purification of the biosurfactant from a promising strain of Staphylococcus saprophyticus, and its biochemical characterization, pH and temperature stability studies and evaluation of the antimicrobial potential of the biosurfactant against different human pathogenic clinical isolates.
Materials and methods
Sample collection
Sediment samples were collected using Petersen grab sampler from four different locations of the petroleum hydrocarbon contaminated coastal sites of Puducherry, India. Possible aseptic techniques were applied while sampling to avoid contamination and the collected samples were transferred to pre-sterilized bottle containers which was kept in an icebox maintained at 4 °C till further processing. The collected samples after reaching the laboratory were processed immediately.
Screening of potential biosurfactant producing bacteria
All the collected four samples were processed individually, in which 1 g of central portions of the samples was serially diluted using sterilized natural seawater (34 ppt) and spread plated on Bushnell Haas agar plates prepared in sea water and supplemented with 1 % crude oil. After 5 days of incubation at 37 °C, individual colonies with distinct morphologies were isolated and further sub-cultured on Zobell marine agar plates to obtain pure cultures, which were maintained in lyophilized form for further studies. All the axenic cultures were individually cultured in Zobell marine broth 2216 for 48 h and the cell free supernatant was used for screening the most promising biosurfactant producers using multiple screening methods, viz., surface activity (Tadros 2005), emulsification activity (Cooper and Goldenberg 1987), lipase activity (Kiran et al. 2010) and oil displacement test (Youssef et al. 2004).
Molecular identification
Molecular identification of the most potential strain was performed based on 16S rRNA gene sequence analysis using the bacterial universal primer set of Eubac 27F (5′-AGAG TTTG ATCM TGGC TCAG-3′) and 1492R (5′-GGTT ACCT TGTT ACGA CTT-3′). The PCR product was purified using the Qiagen PCR purification kit and then sequenced on an ABI Prism 377 automatic sequencer (Applied Biosystems, CA, USA). The 16S rRNA gene sequence from this promising strain was compared with the available bacterial sequences using NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) for their pair-wise identities. Neighbor-joining phylogenetic tree was plotted using the UPGMA statistical method based on the Maximum-Composite-Likelihood model using MEGA 6.0 software (www.megasoftware.net).
Growth kinetics profile as a function of time on biosurfactant production
The identified potential strain was standardized for its peak time of biosurfactant production with reference to its cell growth. The production process was carried out in a 3 L laboratory fermentor (Scigenics, India) with 2.1 L working volume using sea water prepared glucose mineral salt medium as the production medium under the culture conditions of pH 8.0, temperature of 37 °C, 34 ppt salinity, agitation at 150 rpm and aeration at 1.0 vvm. The inoculum was prepared using the exponential phase culture of this promising strain in the same production medium, where the optical density (OD 620 nm) of the inoculum culture was adjusted to 0.1 based on McFarland turbidity 0.5 standards which was equivalent to the bacterial concentration of 1 × 108 cfu/mL. The biosurfactant production was monitored based on the 24 h emulsification index (E 24) (Cooper and Goldenberg 1987) and the bacterial growth was monitored based on the dry weight of cell biomass. The fermentation process was monitored for 96 h and the samples were withdrawn on periodic intervals of 6 h starting from the lag phase to stationary phase under batch culture conditions. The values are represented as mean ± standard deviation of triplicate experiments.
Purification of biosurfactant
At the standardized incubation time, the cell free supernatant was subjected to acid precipitation using 6 N HCl until pH 2 was attained (Nitschke and Pastore 2006). After overnight incubation at 4 °C, the precipitated crude biosurfactant was collected by centrifugation at 5000 rpm for 15 minutes. The obtained crude biosurfactant was neutralized using phosphate buffer (pH 7) and the resultant biosurfactant was extracted with an equal volume of chloroform. The organic phase was separated, concentrated and rotary vacuum evaporated (Lablinks PBU-6, India) which was further purified on normal phase silica gel (60–120 mesh, HiMedia Laboratories Pvt. Ltd., Mumbai, India) column chromatography using stepwise elution with methanol and chloroform ranging from 1:20 to 1:1 (v/v). Twenty fractions were collected and every fraction was screened for the emulsification index (E 24) and the purity of biosurfactant was checked by thin layer chromatography. The fraction(s) showing maximum activity were pooled, rotary evaporated and lyophilized for further studies.
Biochemical characterization
The purified biosurfactant was analyzed on silica gel 60 TLC plate (F254, Merck) which was separated using CH3Cl:CH3OH:H2O (65/15/2, v/v/v) as developing system. Visualizing reagents used were ninhydrin reagent (0.2 g ninhydrin in 100 mL ethanol) to detect peptides, anthrone reagent (1 g anthrone in 5 mL sulfuric acid mixed with 95 mL ethanol) to examine sugars and lipid portion was evidenced using rhodamine B reagent (0.25 g in 100 mL ethanol). Following this, the total content of protein, carbohydrate and lipid was estimated using Lowry’s method (Lowry et al. 1951), phenol sulphuric acid method (Dubois et al. 1956) and total free fatty acids (Folch et al. 1957), respectively. Further, the FT-IR spectrum was used to elucidate the functional groups present in the purified unknown biosurfactant. One milligram of freeze-dried biosurfactant was grounded with 100 mg of KBr. Infrared absorption spectrum were recorded on a Thermo Nicolet, AVATAR 330 FTIR system with a spectral resolution of cm−1 with an average of 10 scans in the wave number range of 400–4000 cm−1, and KBr pellet was used as the background reference.
Molecular mass determination
MALDI-TOF MS was used to examine the molecular mass of the purified biosurfactant. MS was carried out using a Voyager DE-Pro MALDI-TOF spectrometer (Applied Biosystems, Inc, CA, USA) in reflector mode with an accelerating voltage of 20 kV. Equal volume (2 µL) of the purified fraction was mixed with an equal volume of matrix solution, i.e. 0.1 % α-cyano-4-hydroxycinnamic acid in acetonitrile–water–TFA (50:50:0.01, v/v/v). After mixing, the sample was spotted on the target plate, dried, placed inside the sample cabinet and the molecules were separated based on their molecular weight.
Stability studies
Stability of the biosurfactant was evaluated using 24 h emulsification index (E 24) (Sheppard and Mulligan 1987). The emulsification activity was estimated using crude oil as the solvent and the estimations were carried out after exposure for an hour under the specified test conditions. The purified biosurfactant at 4 mg/mL concentration in distilled water was tested for the effect of different temperatures ranging between 30 and 120 °C (Cooper and Goldenberg 1987) and the influence of pH was estimated by adjusting the initial pH of the medium from 2 to 10 (Nitschke and Pastore 2006).
Antimicrobial activity
The antimicrobial activity of the purified biosurfactant was studied on Muller-Hinton agar (MHA) plates against a panel of different human pathogens using antimicrobial disk susceptibility tests as per the Clinical and Laboratory Standards Institute (2015) recommendations. The human bacterial pathogens used were Escherichia coli, Salmonella typhi, S. paratyphi, Klebsiella pneumoniae, K. oxytoca, Vibrio parahemolyticus, V. cholerae, Proteus mirabilis, Streptococcus pneumoniae, Bacillus subtilis, B. cereus and Staphylococcus aureus and human fungal pathogens used were Aspergillus niger, A. flavus, Candida albicans, Cryptococcus neoformans and C. gattii, which were kindly provided by Rajah Muthiah Medical College Hospital, Annamalai University, Tamilnadu, India. These strains were cultured on nutrient broth at 37 °C and the OD of these broth cultures were adjusted to 0.1 equivalent to an inoculum concentration of 108 cfu mL−1 (according to McFarland turbidity standard). MHA plates were swab cultured with 100 µL of individual pathogenic strains and the wells were impregnated with 50 µL of purified biosurfactant dissolved in phosphate buffer (pH 7) at different concentrations (1–128 µg/mL) to obtain the minimum inhibitory concentration (MIC) whereas phosphate buffer solution was used as control. After incubation for 24 h at 37 °C, the plates were examined for zone diameter of inhibition using an antibiotic zone scale.
Results and discussion
Isolation and screening of most promising biosurfactant producing bacterium
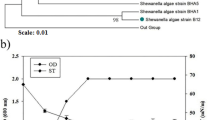
After the specified incubation period, the Bushnell Haas agar plates were examined for distinct morphological colonies which were isolated and pure cultured on Zobell marine agar plates. A total of 51 axenic strains were isolated and named as SBPS 1–51. These isolates were screened to identify the most promising biosurfactant producer(s) using multiple screening tests. Only 11–30 % of the isolates showed potent activity with respect to surface tension reduction (13 %), emulsification activity (11 %), lipase activity (20 %) and oil displacement test (30 %). Among these isolates, only one strain SBPS 15 showed promising activity with respect to all the screening tests, viz., surface tension reduction (32 mN/m), emulsification activity (77.5 %), lipase activity (66 U/mL) and oil displacement (3.3 cm), while the rest of the strains showed highly variable results against the different screening methods. In accordance to the present investigation, Bodour and Maier (2000) earlier reported the significance of petroleum contaminated sites as the best sources for the isolation of promising biosurfactant producers. Many researchers have also previously reported the significance of multiple screening tests for isolation of potential biosurfactant producers and some methods were chosen as standard techniques for the effective isolation of biosurfactant producers (Khopade et al. 2012; Kiran et al. 2010). Based on the multiple screening methods adopted in the present study, strain SBPS 15 was identified as a promising biosurfactant producer. The cell morphology based on Gram staining and microscopic observation revealed the strain SBPS 15 to be Gram-positive cocci. The molecular identification was performed by amplifying the 16S rRNA region and the sequence homology was examined based on BLASTn analysis. The total length of the amplified sequence was 1441 bp. The BLASTn homology comparison of the 16S rRNA gene sequence of the strain SBPS 15 against the nucleotide sequence collection of the NCBI GenBank sequence database showed 100 % sequence similarity with Staphylococcus saprophyticus A6 (accession number KX262676.1). Based on these comparisons, the strain SBPS 15 was identified as Staphylococcus saprophyticus and the 16S rRNA sequence was deposited in NCBI GenBank with the accession number KX352162. The genus Staphylococcus belongs to the family Staphylococcaceae and the phylum Firmicutes. The phylogenetic tree of Staphylococcus saprophyticus SBPS 15 plotted with respect to their homology with NCBI strains is shown in Fig. 1. To the best of our knowledge, Staphylococcus saprophyticus was unexplored for the production and characterization of biosurfactant, hence further studies were undertaken to this regard.
Growth kinetics profile of biosurfactant production
The kinetic profile of biosurfactant production by strain SBPS 15 as a function of time evidenced that the biosurfactant secretion was observed from the early logarithmic phase of bacterial growth and the peak emulsification index (E 24) was measured during the initiation of the stationary growth phase of the bacterium (66th hour). Further, the maximum emulsification activity (E 24) of 77.8 ± 2.3 % was maintained throughout the stationary phase of the bacterium with cell biomass concentration of 8.13 ± 0.35 g/L (Fig. 2). The results revealed that the biosurfactant production with reference to its biomass concentration indicated that they are predominantly produced during the exponential phase and they function as primary metabolites for the normal growth and nutrient uptake, while in the other case they function as secondary metabolite having an ecological role rather than growth, similar to that of antibiotics and pigments (Mulligan et al. 2014). A previous study also reported a similar growth-dependent pattern of biosurfactant production in marine Streptomyces species B3 (Khopade et al. 2012).
Purification and characterization of the biosurfactant
From the cell free supernatant, the crude biosurfactant was recovered by acid precipitation, extracted and further purified using silica gel column chromatography. The maximum emulsification activity quantified in methanol:chloroform (1:11) eluted fraction was 1.345 ± 0.056 g/L on dry weight basis. The silica gel TLC plate analysis of the purified biosurfactant revealed the presence of two spots corresponding to lipid and carbohydrate with Rf values of 0.73 and 0.45. The carbohydrate and lipid contents estimated were 738 mg/g (nearly 74 % of carbohydrates) and 262 mg/g (nearly 26 % of lipid) in the biosurfactant based on biochemical estimation methods and protein was not detected. The purified biosurfactant was further analyzed for its functional groups based on FT-IR spectrum (Fig. 3a). The presence of characteristic adsorption bands at 864, 1386, 2862 and 2926 cm−1 revealed the presence of aliphatic long fatty acid chain (Guo et al. 2009; Rahman et al. 2010). The important functional groups, viz., OH bond (3421 cm−1), alkene (C = C) (1643 cm−1) and carbonyl group (C–O) (1182 cm−1) were the characteristic groups present in the biosurfactants (Pornsunthorntawee et al. 2008; Rahman et al. 2010). The presence of the more important band at 1724 cm−1 deduced the presence of carboxyl group, which represented the linkage group between the sugar and fatty acid (Rodrigues et al. 2006) and vibration at 1105 cm−1 was reported as corresponded to the C–O–C stretching, which predicts the presence of sugar moiety (Pornsunthorntawee et al. 2008). These results suggest that the isolated biosurfactant belongs to the family of glycolipids.
In support to the current prediction, prior studies have reported that microbial strains from the same species isolated from different ecological niches produce different types of biosurfactant, for example, Serratia marcescens isolated from terrestrial habitats produced Serrawettin, an exolipid biosurfactant (Li et al. 2005); however, Dusane et al. (2011) reported a glycolipid biosurfactant produced by S. marcescens isolated from marine habitats. Likewise, Pseudomonas aeruginosa is mostly reported to produce rhamnolipids, a glycolipid biosurfactant (Pornsunthorntawee et al. 2008); however, a lipopeptide biosurfactant was produced by P. aeruginosa isolated from sea water of Tuticorin Harbor, India (Thavasi et al. 2011). Further, in the present investigation, the isolated biosurfactant from the marine bacterium S. saprophyticus revealed an interesting observation in its biochemical diversity, producing a glycolipid biosurfactant which was unique as compared to an earlier report of Staphylococcus sp. strain 1E isolated from terrestrial hydrocarbon contaminated soil of Algeria which produced a lipopeptide biosurfactant (Eddouaouda et al. 2012). In addition, many researches have earlier evidenced that marine microbes represent a distinctive group owing to their immense genetic (Sogin et al. 2006) and biochemical diversity (Rusch et al. 2007), and a rich resource for a wide range of bioactive compounds (Debbab et al. 2010).
The molecular weight of the purified glycolipid biosurfactant from strain SBPS 15 was examined using MALDI-TOF–MS analysis. The spectral analysis revealed the presence of a cluster of three molecules at m/z 607.7, 629.7 and 645.7 which were attributed to the molecules of protonated ion and to the adducts of sodium and potassium ions (Fig. 3b). Based on the above observations, the molecular mass of this glycolipid biosurfactant is 606.7 Da and its molecular weight was found to be different from the earlier reported glycolipid biosurfactants and a new addition to the existing list of glycolipid biosurfactants. This result showed good arguments with Abdel-Mawgoud et al. (2010) who suggested that the molecular mass of most reported glycolipid biosurfactants are present within the molecular mass range of 302–803 Da.
Stability studies
Emulsification index (E 24) was used for measuring the biosurfactant stability. The purified glycolipid biosurfactant from strain SBPS 15 showed increased emulsification (E 24 of 82 %) in its pure form as compared to the crude form observed during screening (E 24 of 77.5 %). It showed good stability over a wide range of pH 3–9 with E 24 of 72–80 % and significantly reduced its activity beyond pH 9 and above pH 3 (Fig. 4a). Similar results were observed in case of an emulsifier produced by the yeast Yarrowia lipolytica NCIM 3589 cultivated in n-hexadecane (Sobrinho et al. 2008). In terms of temperature stability, the glycolipid biosurfactant from strain SBPS 15 showed no considerable loss of activity up to 80 °C (E 24 of 72–80 %); however, beyond this temperature, it started to denature and showed no emulsification activity at 100 °C (Fig. 4b). On the other hand, Makkar and Cameotra (2002) reported that the biosurfactant produced by the bacterial isolates were stable at higher temperature up to 120 °C. The broad stability range observed for this biosurfactant makes it a suitable candidate for many desired applications which mandates a stable emulsion at different physico-chemical conditions.
Antimicrobial activity
Among the twelve tested bacterial human pathogens, the purified biosurfactant from strain SBPS 15 showed antimicrobial activity against seven strains. The maximum zone of inhibition and the respective minimum inhibitory concentration (MIC) values recorded were Klebsiella pneumoniae (23 mm, 4 µg/mL) followed by Escherichia coli (20 mm, 12 µg/mL), Pseudomonas aeruginosa (20 mm, 32 µg/mL), Vibrio cholerae (18 mm, 64 µg/mL), Bacillus subtilis (15 mm, 48 µg/mL), Salmonella paratyphi (13 mm, 32 µg/mL) and Staphylococcus aureus (11 mm, 12 µg/mL) (Table 1). Among the tested pathogens, the inhibitory activity was observed in case of both Gram-positive as well as Gram-negative strains with minimal MIC values which show its promising and diverse antimicrobial potential.
Regarding the antifungal activity against the tested five different fungal human pathogens, the biosurfactant from strain SBPS 15 showed activity against three strains with maximum zone of inhibition and the respective MIC values recorded were Cryptococcus neoformans (22 mm, 32 µg/mL) followed by Candida albicans (21 mm, 32 µg/mL) and Aspergillus niger (15 mm, 16 µg/mL) (Table 1). Based on these studies, it was observed that the isolated biosurfactant showed a board range of potential antimicrobial activity against both the bacterial and fungal pathogenic strains. According to Das et al. (2008), lipopeptide surfactants are largely reported for wide antimicrobial activities and relatively less number of reports exists on glycolipid biosurfactants exhibiting effective antimicrobial activity. Similar to the present investigation, the glycolipid biosurfactant produced from marine Streptomyces species B3 isolated from west coastal sediment of India showed antimicrobial activities towards different human pathogens (Khopade et al. 2012).
Conclusions
In the present study, the isolated biosurfactant from the marine S. saprophyticus SBPS 15 showed significant surface active properties such as surface tension reduction, emulsification, lipase and oil displacement activities. The purified biosurfactant showed a different molecular mass as compared to the earlier reports which can be designated as a new glycolipid biosurfactant. Further, the purified glycolipid biosurfactant revealed a board range of pH and temperature stability which suggests its significance for possible application in diverse niche areas. Moreover, the excellent antibacterial and antifungal activities of this biosurfactant against a board spectrum of clinical human pathogens evidenced its importance in the field of therapeutics. Taking all together, the isolated new glycolipid biosurfactant from this marine strain exhibited dual functions as a surface-active and antimicrobial agent which qualifies it as a promising candidate for possible use in biomedical applications.
References
Abdel-Mawgoud AM, Lepine F, Deziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Ahimou F, Jacques P, Deleu M (2000) Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb Technol 27:749–754
Arutchelvi JI, Bhaduri S, Uppara PV, Doble M (2008) Mannosylerythritol lipids: a review. J Ind Microbiol Biotechnol 35:1559–1570
Benincasa M, Abalos A, Oliveira I, Manresa A (2004) Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 85:1–8
Bodour AA, Maier M (2000) Biosurfactants: Types, screening methods and applications. In: Bitton G (ed) Encyclopedia of environmental microbiology, 1st edn. Wiley, Hoboken, pp 750–770
Campos JM, Stamford TLM, Sarubbo LA, Luna JM, Rufino RD, Banat IM (2013) Microbial biosurfactants as additives for food industries. Biotechnol Prog 29:1097–1108
Cavalero DA, Cooper DG (2003) The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J Biotechnol 103:31–41
Clinical and Laboratory Standards Institute (2015) Performance standards for antimicrobial susceptibility testing. In: 25th Informational Supplement Document M100-S25, CLSI, Wayne
Cooper D, Goldenberg B (1987) Surface active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol 104:1675–1684
Debbab A, Aly AH, Lin WH, Proksch P (2010) Bioactive compounds from marine bacteria and fungi. Microb Biotechnol 3:544–563
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dusane DH, Pawar VS, Nancharaiah YV, Venugopalan VP, Kumar AR, Zinjarde SS (2011) Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 27:645–654
Eddouaouda K, Mnif S, Badis A, Younes SB, Cherif S, Ferhat S, Mhiri N, Chamkha M, Sayadi S (2012) Characterization of a novel biosurfactant produced by Staphylococcus sp. strain 1E with potential application on hydrocarbon bioremediation. J Basic Microbiol 52:408–418
Fang Y, Lu Z, Lv F, Bie X, Liu S, Ding Z, Xu W (2006) A newly isolated organic solvent tolerant Staphylococcus saprophyticus M36 produced organic solvent-stable lipase. Curr Microbiol 53:510–515
Fenical W (1993) Chemical studies of marine bacteria: developing a new resource. Chem Rev 93:1673–1683
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gudina EJ, Teixeira JA, Rodrigues LR (2016) Biosurfactants produced by marine microorganisms with therapeutic applications. Mar Drugs 14:E38. doi:10.3390/md14020038
Guo Y, Hu Y, Gu RR, Lin H (2009) Characterization and micellization of rhamnolipidic fractions and crude extracts produced by Pseudomonas aeruginosa mutant MIG-N146. J Colloid Interface Sci 331:356–363
Khopade A, Ren B, Liu X, Mahadik K, Zhang L, Kokare C (2012) Production and characterization of biosurfactant from marine Streptomyces species B3. J Colloids Interface Sci 367:311–318
Kiran GS, Thomas TA, Selvin J (2010) Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Coll Surf B Biointerf 78:8–16
Li H, Tanikawa T, Sato Y, Nakagawa Y, Matsuyama T (2005) Serratia marcescens gene required for surfactant serrawettin W1 production encodes putative aminolipid synthetase belonging to nonribosomal peptide synthetase family. Microbiol Immunol 49:303–310
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Makkar RS, Cameotra SS (2002) An update on the use of unconventional substrates for biosurfactants production and their new applications. Appl Microbiol Biotechnol 58:428–434
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Mulligan CN, Mudhoo A, Sharma SK (2014) Biosurfactants: research trends and applications. CRC Press, Boca Raton
Nitschke M, Pastore GM (2006) Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol 97:336–341
Popowicz GM, Dubin G, Stec-Niemczyk J, Czarny A (2006) Functional and structural characterization of Sp1 protease from Staphylococcus aureus. J Mol Biol 358:270–279
Pornsunthorntawee O, Wongpanit P, Chavadej S, Abe M, Rujiravanit R (2008) Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum-contaminated soil. Bioresour Technol 99:1589–1595
Proksch P, Edrada-Ebel R, Ebel R (2003) Drugs from the sea—opportunities and obstacles. Mar Drugs 1:5–17
Rahman PKSM, Pasirayi G, Auger V, Ali Z (2010) Production of rhamnolipid biosurfactant by Psuedomonas aeruginosa DS 10-129 in a microfluidic bioreactor. Biotechnol Appl Biochem 55:45–52
Rodrigues LR, Teixeira JA, Mei HCV, Oliveira R (2006) Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis. Colloids Surf B 49:78–85
Ron EZ, Rosenberg E (2002) Biosurfactants and oil bioremediation. Curr Opin Microbiol 13:249–252
Ruggeri C, Franzetti A, Bestetti G, Caredda P, Colla PL, Pintus M, Sergi S, Tamburini E (2009) Isolation and characterization of surface active compound producing bacteria from hydrocarbon-contaminated environments. Int Biodeterior Biodegrad 63:936–942
Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S, Wu D, Elsen JA, Hoffman JM, Remington K, Beeson K, Tran B, Smith H, Baden-Tillson H, Stewart C, Thorpe J, Freeman J, Andrews-Pfannkoch C, Venter JE, Li K, Kravitz S, Heidelberg JF, Utterback T, Rogers Y-H, Falcón LI, Souza V, Bonilla-Rosso G, Eguiarte LE, Karl DM, Sathyendranath S, Platt T, Bermingham E, Gallardo V, Tamayo-Castillo G, Ferrari MR, Strausberg RL, Nealson K, Friedman R, Frazier M, Venter JC (2007) The sorcer 11 global ocean sampling expedition: north-west Atlantic through Eastern Tropical Pacific. PLoS Biol 5:e77. doi:10.1371/journal.pbio.0050077
Sachdev DP, Cameotra SS (2013) Biosurfactants in agriculture. Appl Microbiol Biotechnol 97:1005–1016
Sheppard JD, Mulligan CN (1987) The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl Microbiol Biotechnol 27:110–116
Singh P, Cameotra SS (2004) Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol 22:142–146
Sobrinho HBS, Rufino RD, Luna JM, Salgueiro AA, Takaki GMC, Leite LFC, Sarubbo LA (2008) Utilization of two agro industrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem 43:912–917
Sogin ML, Morrison HG, Huber JA, Welch DM, Huse SM, Neal PR, Arrieta JM, Herndl GJ (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci USA 103:12115–12120
Tadros T (2005) Adsorption of surfactants at the air/liquid and liquid/liquid interfaces. In: Applied surfactants: principles and applications. Wiley, Weinheim, pp 81–82
Thavasi R, Nambaru VRMS, Jayalakshmi S, Balasubramanian T, Banat IM (2011) Biosurfactant production by Pseudomonas aeruginosa from renewable resources. Indian J Microbiol 51:30–36
Youssef NH, Dunacn KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods 56:339–347
Zhang L, Somasundaran P, Singh SK, Felse AP, Gross RA (2004) Synthesis and interfacial properties of sophorolipid derivatives. Colloids Surf A Physicochem Eng Asp 240:75–82
Acknowledgments
The authors very gratefully acknowledged Shri R. J. Antony Raj, Chairman and Mrs. A. Agnes Antony Raj, Secretary, SIMPRA, SIRO Institution, Thanjavur, Tamil Nadu, India, for providing lab facilities and supporting our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest on publication of this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mani, P., Dineshkumar, G., Jayaseelan, T. et al. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech 6, 163 (2016). https://doi.org/10.1007/s13205-016-0478-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-016-0478-7