Abstract
Pseudomonas aeruginosa is a bacterium producing industrially utile metabolites, such as rhamnolipids, biopolymers, and pigments. Pyocyanin, the most studied example of pigments, is a virulence factor that also shows the potential for application in, e.g., agriculture, anticancer therapy, and energy production. Therefore, potential inhibitors and stimulants of pyocyanin production by P. aeruginosa should be studied, and nanomaterials may cause both effects. The study aimed to examine the influence of zinc oxide and multi-walled carbon nanotubes (pristine or dispersed with alginic acid) on pyocyanin production by P. aeruginosa. First, the influence of different concentrations of nanomaterials (500.00–0.06 µg/mL) on culture optical density and biofilm formation was studied. These results helped select concentrations for further tests, i.e., growth curves and fluorescence measurements. Pyocyanin production was assessed by the chloroform–hydrochloric acid method. SEM analysis was conducted to assess the influence of nanomaterials on the cell's integrity and biofilm structure. Pristine multi-walled carbon nanotubes exhibited a stimulative effect on pigment production when applied in high concentrations (500.00 µg/mL), while dispersed material enhanced the production in lowered dosages (125.00 µg/mL). On the other hand, high concentrations of zinc oxide inhibited pyocyanin production, while minor increased bioproduct production. The research indicates the potential to use nanomaterials as the modulators of pyocyanin production and other metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, nanomaterials have gained more and more attention due to their application potential in different branches of industry, e.g., electronics, photocatalysis, environment protection, and civil engineering (Sikora et al. 2016; Lah et al. 2018; Kolahalam et al. 2019; Markowska-Szczupak et al. 2020). Some of them exhibit antimicrobial properties and, therefore, are used to eradicate pathogenic microorganisms.
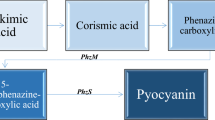
Pseudomonas aeruginosa is an opportunistic pathogen belonging to the ESKAPE group, often associated with nosocomial infections and complications in cystic fibrosis patients (Hall et al. 2016; Grygorcewicz et al. 2020). Therefore, many researchers focus on eliminating P. aeruginosa employing different nanomaterials, mainly metal oxides (Lee et al. 2014; Zanni et al. 2017; Saleh et al. 2019; El-Deeb et al. 2020; Ali et al. 2020; Najafi et al. 2021) However, it was reported that Pseudomonas spp. biofilm formation or cell respiration can be stimulated by nanomaterials, such as silver nanoparticles, zinc oxide, and hybrid nanomaterial consisting of silica nanotubes modified with titanium dioxide (Yang and Alvarez 2015; Augustyniak et al. 2020; Ouyang et al. 2020). Following these findings, it may be hypothesized that nanomaterials can also influence the production of bacterial pigments. A well-known example of the pigment produced by P. aeruginosa is pyocyanin belonging to phenazines. Pyocyanin is a blue–greenish product recognized as a virulence factor. Its presence can be problematic, especially in the case of P. aeruginosa infections, as it is a source of reactive oxygen species that, while present in excess, may lead to oxidative stress at the infection site (Hall et al. 2016). However, due to the distinct properties of pyocyanin, it shows great potential to be applied in many technologies, e.g., microbial fuel cells (Shen et al. 2014), OLED screens (Chen and Li 2004), sensors (Yu et al. 2017), agriculture (Meena et al. 2020a), cancer and mitochondrial disease treatments (Zhao et al. 2014; Moayedi et al. 2018; Kohatsu et al. 2020; Peruzzo et al. 2021), environmental protection (Viana et al. 2018) and phage conversion (Jancheva and Thomas 2021). However, pyocyanin remains a costly reagent (e.g., €118 for 5 mg on Merck website). Therefore, the production of pyocyanin is optimized by many research groups by adjusting process conditions, genetic engineering of the microorganisms, or the addition of various substances to the medium, including nanomaterials (Sismaet et al. 2014; El-Fouly et al. 2015; Ozdal 2019; Ozdal et al. 2019; Bacame-Valenzuela et al. 2020; da Silva et al. 2021; Elbargisy 2021). The analysis of the literature shows that most of the data concerning the influence of nanomaterials on pyocyanin production indicate the inhibition of pigment production. However, the concentrations used are relatively high, especially in the case of metal oxide nanoparticles. Moreover, the data about the use of different types of nanomaterials, e.g., carbon nanomaterials, to influence pyocyanin production appears to be insufficient.
Therefore, the study aimed to investigate the influence of a wide range of concentrations of zinc oxide nanoparticles and multi-walled carbon nanotubes on pyocyanin production, establish the optimal culture conditions, and assess their influence on the basic physiology of P. aeruginosa.
Materials and methods
Materials
Nanomaterials and materials used in the research are commercially available and were purchased at Sigma Aldrich (Darmstadt, Germany). Zinc oxide nanoparticles were previously characterized by Augustyniak et al. (2021). Multi-walled carbon nanotubes were characterized employing XRD and SEM–EDS (Supplementary materials, Figs. S1 and S2). Nanomaterials were prepared for biological tests by suspending in ultrapure water, heated to 100 °C for 15 min to prevent contamination in further experiments, and later placed in an ultrasonic bath for 1 h (with coolers to prevent heating of the materials). Regarding MWCNT, an additional suspension with alginic acid (AA, in ratio MWCNT:AA equal to 1:1) was prepared to obtain better dispersion, as suggested by Wang et al. (2018). The bacterial strain used in the research was Pseudomonas aeruginosa ATCC®27853™. Bacteria were kept at − 20 °C in TSB medium supplemented with 10% glycerol and revived on TSA medium before the experiments.
The influence of different nanomaterials concentrations on optical density and biofilm formation
The influence of the nanomaterials on the optical density of P. aeruginosa culture was assessed with spectrophotometer BioTek Synergy H1 (BioTek Instruments, Winooski, VT, USA). P. aeruginosa overnight culture was prepared by suspending one colony in a sterile TSB medium and incubating at 37 °C for 16 h. Then, the culture was diluted in ratio 1:100 in fresh King A medium (2 \(\times\) concentrated to avoid changes in nutrients concentration). The experiment was conducted in 96-well polystyrene plates. Each well was filled with 50 µL of the nanomaterial in the chosen concentration and 50 µL of diluted culture. The range of tested concentrations varied from 500 to 0.06 µg/µL for both MWCNT and ZnO nanoparticles to cover a wide range of tested concentrations. The optical density (λ = 600 nm, measurements in 9 points of each well) was measured after 48 h of incubation. Afterward, a biofilm assay was carried out according to the methodology presented by Sikora et al. (2018). The wells were washed thrice with PBS buffer, air-dried, and the biofilm was fixed with methanol for 15 min. Next, the biofilm was stained with 0.1% crystal violet solution for 15 min and washed with deionized water. Ethanol/acetone solution (in ratio 4:1) was added to the wells to determine biofilm biomass by absorbance measurement at λ = 595 nm. Both parameters were measured on BioTek Synergy H1 (BioTek Instruments, Winooski, VT, USA). In the experiments, bacterial culture without nanomaterial addition was a positive control. The experiments were conducted in three separate replicates.
Growth curves and fluorescence measurements
The experiment was conducted in 24-well polystyrene plates for 16 h. The optical density measurements (λ = 600 nm, measurements in 9 points of each well) and fluorescence (λex = 485 nm λem = 516 nm for general fluorescence and λex = 398 nm λem = 460 nm for pyoverdine (Mohanty et al. 2015) were carried out every 30 min.
Culture conditions for pyocyanin production
The first step of the research concerning pyocyanin production was the choice of process conditions without exposure to nanomaterials. Parameters such as the volume of the culture, interphase area, and agitation were evaluated. Moreover, the King A medium that was proven effective for pyocyanin production was used as a culture medium (Viana et al. 2018; Debritto et al. 2020; Elbargisy 2021). The experiments were conducted in three types of vials—Falcon-like tubes (50 mL), conical flasks (250 mL), and Petri dish (\(\varnothing\)=90 mm). Regarding Falcon-like tubes and flasks, five different culture volumes were tested (10, 20, 30, 40, and 50 mL). The volume tested in the case of the Petri dish was 10 mL (higher volumes were not tested due to the limited capacity of the Petri dish) and was not subjected to agitation considering the potential risk of the spill. Moreover, all the other setups were tested with (100 rpm, orbital shaking) and without agitation. After 48 h of incubation, samples were extracted employing the chloroform–hydrochloric acid method described elsewhere (El-Fouly et al. 2015). The experiments were conducted in two separate replicates. The results are shown as a ratio of pyocyanin production (Eq. 1) in the tested vial to pyocyanin production in 30 mL of culture conical flask (E30A) with agitation (treated as a control as it is our standard way of preparing the culture):
where RPYO is the pyocyanin production ratio, \({{(C}_{\mathrm{PYO}})}_{\mathrm{vial}}\) is the pyocyanin production by culture incubated in selected process conditions, \({{(C}_{\mathrm{PYO}})}_{E30A}\) is the pyocyanin production in 30 mL of culture conical flask with agitation.
The influence of nanomaterials on pyocyanin production
Based on the previously conducted assay results, the experiments were carried out in the Petri dish and covered the most promising concentrations of nanomaterials—500.00, 125.00, 31.25, and 7.81 µg/mL. The diluted overnight culture was incubated with the chosen nanomaterial concentration for 48 h. After that, extraction was performed employing chloroform–hydrochloric acid assay based on the methodology proposed by Essar et al. (1990). Briefly, the cultures were centrifuged after 48 h of incubation. Five mL of the supernatant was mixed with 3 mL of chloroform to extract pyocyanin (blue phase). Later, pyocyanin was reextracted with 0.2 N HCl (pink phase) and subjected to absorbance measurement at wavelength λ = 520 nm. The concentration of pyocyanin in 1 mL of the medium was calculated using the calibration curve of the HLPC-grade standard. The experiment was conducted in triplicate. The results are presented as pyocyanin production index (IPYO, Eq. 2):
where IPYO is the pyocyanin production index, \({{(Y}_{\mathrm{PYO}})}_{\mathrm{nanomaterial}}\) is the pyocyanin production by culture incubated with nanomaterial, \({{(Y}_{\mathrm{PYO}})}_{\mathrm{control}}\) is the pyocyanin production by the control culture.
SEM analysis
Biofilm structure and cell morphology after incubation with ZnO, MWCNT, or MWCNT-AA were examined by scanning electron microscopy. The samples were prepared in two ways: (1) by placing a sterile cover glass in a Petri dish filled with freshly prepared culture for 24 h, (2) by collecting a 24-h-long liquid culture with the nanomaterial and placing it on the cover glass. The first sample type was prepared to observe the biofilm formation in the cultures that exhibited significantly lowered or increased pyocyanin production (500 and 7 µg/mL for ZnO, 500 µg/mL for pristine MWCNT, 500 and 125 µg/mL for MWCNT-AA). Another sample type was produced to observe the influence of 24-h-long culture on nanomaterials (samples with 500 µg/mL of each nanomaterial to ensure that nanomaterials will be visible in the sample). The samples were subjected to the glutaraldehyde–ethanol protocol to remove the water from the cells without changing their morphology (Soler-Arango et al. 2019). Afterward, the samples were sputtered with chromium, since the biological material was not conductive.
Statistical analysis
Obtained results were analyzed employing one-way ANOVA with the Tukey test. The results were considered significantly different at p < 0.05.
Results and discussion
The influence of different nanomaterials concentrations on optical density and biofilm formation
All ZnO nanoparticles (Fig. 1a) concentrations did not influence the optical density after 48 h of incubation. Moreover, biofilm biomass did not differ from the control sample after incubation with all applied ZnO concentrations (Fig. 1b). The same observations were true for the pristine MWCNT sample (Fig. 1c), which did not significantly influence optical density and biofilm biomass. However, for MWCNT dispersed with AA, higher optical density was noted for concentrations ranging from 500.00 to 15.63 µg/mL (Fig. 1e). In addition, lower biomass was observed for concentrations 500.00 and 31.25 µg/mL (Fig. 1f). It is worth mentioning that the p value for concentrations 250.00–62.50 µg/mL was only slightly above 0.05 (p value equal to 0.0557, 0.0513 and 0.0563, respectively). An additional control sample containing AA was included to confirm that observed phenomena are not based on the presence of AA alone. It showed that this dispersing agent did not significantly influence any of the tested parameters (Fig. 1g and h). Hence, it was proven that noted differences could be assigned to dispersed MWCNT.
Optical density and biofilm biomass of P. aeruginosa culture exposed to different concentrations of ZnO (a and b), MWCNT (c and d), MWCNT with AA (e and f), and AA (g and h), respectively. Error bars indicate SEM (standard error of the mean). Statistically significant differences (p < 0.05, ANOVA, Tukey test) from the control sample are indicated with *
Moreover, the visual inspection of the experiment after 48 h of incubation revealed variability in the colors of the cultures, which varied from yellow–green to blue–green (Supplementary materials, Fig. S3). It was noticed that in the case of MWCNT-AA, the five highest concentrations (500.00–31.25 µg/mL) expressed high blue–greenish pigment production. However, in the case of MWCNT without AA, only a concentration of 500 µg/mL led to the production of blue–greenish pigment. Regarding ZnO nanoparticles, it was noted that cultures became slightly more blue–greenish while incubated with concentrations ranging from 31.25 to 7.81 µg/mL. AA control assay was characterized by yellow–greenish color with no observable differences between the applied concentrations (data not shown).
Based on the obtained results and visual observations, further assays were conducted with four chosen nanomaterial concentrations (500.00, 125.00, 31.25, and 7.81 µg/mL) to present the variability of the obtained effects.
Obtained results showed that ZnO, pristine MWCNT, and AA did not change the optical density of the culture and its biofilm biomass. However, dispersed MWCNT did significantly change both parameters. We hypothesize that higher optical density reads may result from the observed color change of the culture due to pigment production. Concerning lower biofilm biomass, bacteria could have covered the nanomaterial that was later removed during the biofilm staining procedure. Kovach et al. (2020) described such phenomenon when observing aggregation of P. aeruginosa while incubated with acid-functionalized MWCNT in a relatively small concentration of 2 µg/mL. The same behavior was reported after incubation of P. aeruginosa PAO1 with UV-activated silica–titania nanocomposite (Augustyniak et al. 2020). However, aggregation of the cells was also observed even when the nanomaterial was not exposed to UV light.
Growth curves
The growth curves assay was conducted with chosen nanomaterial concentrations (500.00, 125.00, 31.25, and 7.81 µg/mL). In the case of 500.00 µg/mL of ZnO nanoparticles, the concentration exceeded the possibility to compensate for the background noise. The initial optical density of the culture was too high, which prevented the accurate assessment of the growth of P. aeruginosa (data not shown). Perhaps, other methods such as plate count should be applied instead. Nevertheless, the assay was successfully carried out with the remaining concentrations. It was noted that 125.00, 31.25, and 7.81 µg/mL of ZnO inhibited the growth of the culture after around 6 h (that can be interpreted as a logarithmic phase of growth) in comparison to the control sample. It is worth underlining that such differences were not noted in the previous assay (OD measurement after 48 h), indicating that nanomaterials influence the growth of the culture only in the logarithmic and early stationary phases. Regarding pristine MWCNT, optical density measurements showed the decreased values of culture incubated with 500.00 µg/mL of MWCNT and no pronounced changes in the case of three lower ones (Fig. 2b). It is worth mentioning that OD values for concentrations 500.00 and 125.00 were characterized with relatively high standard error of the mean, which might result from the hydrophobic nature and agglomeration of MWCNT that cause problems with the dispersion and replicable measurements (Wang et al. 2018). For that reason, the number of repetitions of each case was increased in our investigation. On the other hand, growth curves with MWCNT-AA showed that all tested nanomaterial concentrations lead to higher OD values than the control sample (Fig. 2c). The lowest concentration led to a slight increase, while higher concentrations influenced the OD more pronouncedly. The main differences appeared after 7–8 h of incubation, which confirms (similarly to ZnO assay) that the growth changes occur in the late logarithmic phase. In this case, the same observations were made in the previous assay based on OD measurements after 48 h. Concerning the assay conducted only to assess whether AA influences the growth of bacteria, a slight drop in OD measurements was noted for all tested concentrations only from around the sixth to the tenth hour of incubation. It is worth mentioning that in assays covering MWCNT and AA, the most prominent standard error bars are seen in the late phase of the growth. Perhaps, it can be associated with producing other metabolites, e.g., polysaccharides or pigments, by P. aeruginosa that may interfere with OD measurements (Myers et al. 2013).
Additional fluorescence measurements for general fluorescence and pyoverdine monitoring were carried out and presented in Supplementary materials (Figs. S4 and S5). The results showed that all ZnO samples showed higher fluorescence values than the control sample in the case of general fluorescence. The highest signal was noted for concentration equal to 31.25 µg/mL (Fig. S4). Concerning MWCNT, fluorescence was slightly lowered for all tested concentrations besides 500.00 µg/mL. It resulted in decreased signal in comparison to the control. MWCNT-AA samples showed a tendency—the higher the concentration used, the lower the fluorescence values. AA sample with a 500 µg/mL concentration slightly lowered the values. Based on the obtained results, it can be hypothesized that MWCNT may lower the production of fluorescent metabolites (other than pyoverdine), while ZnO heightens it. Pyoverdine assay (Fig. S5) showed a contradictory tendency for ZnO nanoparticles than general fluorescence assay—the highest concentration led to the most significant decrease in fluorescence values. Regarding MWCNT, all tested concentrations lowered pyoverdine production (again, the higher the concentration, the lower fluorescence values). Interesting results were obtained with MWCNT-AA samples. The concentrations of 500.00 and 125.00 µg/mL were on the level of the pure medium signal, while 7.81 and 31.25 µg/mL lowered the fluorescence compared to the control. AA sample showed no differences in the fluorescence values. However, around the 14th hour of the culture, a subtle fluorescence signal appeared, while the sample's fluorescence with 500 µg/mL of AA declined. Obtained results suggest that ZnO nanoparticles and MWCNT may influence the production of pigments other than pyocyanin. Mohanty et al. (2015) conducted research showing that single-walled carbon nanotubes (SWCNT) inhibit pyoverdine production in 40 and 80 µg/mL concentrations. Our results confirmed this tendency with MWCNT that has been recognized as generally less toxic than SWCNT (Kavosi et al. 2018; Chen et al. 2019). On the other hand, Lee et al. (2014) showed that zinc oxide nanoparticles lead to increased pyoverdine production. The outcome obtained in our research contradicts these findings. Nevertheless, it should be emphasized that we used a different pyoverdine quantification method (Lee et al. 2014 used absorbance at 405 nm) and another strain of P. aeruginosa strain.
The influence of culture conditions on pyocyanin production
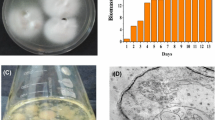
Optimizing the culture conditions (without incubation with the nanomaterials) revealed that all tested parameters influenced pyocyanin production (Fig. 3). First, the greater the culture volume, the lower the pyocyanin production was obtained. Moreover, we have found that the pigment production was also affected by the type of vial used for cultures, indicating that spatial organization of the cultures should be considered. The production was promoted in carriers with a larger air–water interphase area and a thinner liquid layer. The highest production was obtained in the Petri dish (more than 220 times higher than in control), followed by conical flasks and Falcon-type tubes. Obtained results may suggest that the low ratio of the culture volume to the interphase area can promote pyocyanin production. In addition, agitation led to lower pyocyanin production in all tested setups. The difference between agitated cultures and those kept in a steady position was the most visible in the case of conical flasks. The color of the agitated cultures was yellow–greenish, which may indicate the enhanced production of different pigments, e.g., pyoverdine. This result was surprising in the scope of investigations presented by Elbargisy (2021), who obtained higher pigment production in the agitated cultures. However, the agitation conditions in these experiments were set to 200 rpm, whereas 100 rpm was applied in our studies. Another factor contributing to such discrepancy could be the variability between P. aeruginosa strains used in the experiments. Nevertheless, Cabeen (2014) showed intense pyocyanin production in static cultures, corroborating our findings. Complete results of the statistical analysis are presented in the Supplementary materials (Table S1).
The influence of nanomaterials on pyocyanin production
The assay showed that ZnO nanoparticles and MWCNT have different influences on pyocyanin production (Fig. 4). The higher the used concentration, the lower pyocyanin production was obtained concerning ZnO. On the other hand, ZnO nanoparticles in 7.81 µg/mL concentration significantly increased pigment concentration. We hypothesize that ZnO nanoparticles provided to cells in sublethal concentrations could serve as zinc cations donors, leading to increased phenazine production. Zinc cations influence the biochemical processes that lead to elevated pyocyanin production, as they are crucial in enzymatic reactions and even the production of some virulence factors. It was noted that zinc sulfate increases PCA production, a precursor of pyocyanin synthesis (Slininger and Jackson 1992). However, Dieppois et al. (2012) reported that zinc activates the CzsS/CzcR system that represses pyocyanin production. Moreover, zinc cations have reported bacteriostatic and bactericidal activity, which indicates a certain tolerance of this element that can be provided by supplementing the medium with ZnO nanoparticles (Mishra et al. 2017; Siddiqi et al. 2018; Tiwari et al. 2018; Gudkov et al. 2021). In this scope, the inhibition of growth (Fig. 2a) can be interpreted as the bacteriostatic influence of ZnO nanoparticles on P. aeruginosa.
Pristine MWCNT stimulated pyocyanin production in a concentration of 500 µg/mL, while the other applied concentrations did not change the production. MWCNT dispersed with AA improved pyocyanin production, while present in 125.00 and 500.00 µg/mL concentrations. However, AA alone also elevated pyocyanin production, while present at a concentration of 500 µg/mL. Nevertheless, other tested concentrations of AA did not significantly change pigment production.
Our findings show that AA concentrations can be used to improve MWCNT dispersion only in limited concentrations (in our case up to 125 µg/mL) that do not alter pyocyanin production. Higher concentrations may change pyocyanin production, because AA is one of the main biopolymers produced during biofilm formation by Pseudomonas spp. (Laverty et al. 2014; Meena et al. 2020b). It is also considered P. aeruginosa virulence factor, likewise pyocyanin (Jurado-Martín et al. 2021). Perhaps, a high AA concentration may change the signaling pathway that results in increased pyocyanin production. It has been shown that alginate (which is also a natural product of P. aeruginosa) may interfere with PQS signaling reducing rhamnolipids production and promoting intraspecies co-existence in cystic fibrosis patients (Limoli et al. 2017). Nevertheless, simultaneous use of 125 µg/mL of MWCNT and AA leads to the highest stimulation of pyocyanin production, even though none of the agents has the same effect alone. In our opinion, the use of 125 µg/mL MWCNT-AA allows obtaining the same stimulative effect as 500.00 µg/mL of pristine MWCNT. This finding can positively influence the design of the production process, because it allows reducing necessary nanomaterial concentration and eliminating the problems with poor dispersion and low repeatability of pipetting (Ma et al. 2010) (Fig. 4).
SEM analysis
Scanning electron microscopy analysis revealed some differences in the distribution of nanomaterial in two types of prepared samples. In samples with MWCNT and MWCNT-AA, the biofilm seemed undisturbed (in comparison to the control, Fig. 5a), and some carbon nanotubes were covered with cells or incorporated in the biofilm structure (Fig. 5b–d). The samples collected from the liquid cultures showed that single MWCNT were surrounded by cells (Fig. 6a). Concerning MWCNT-AA, the cells and nanotubes seemed to be covered in a thicker layer of an extracellular polymer substance (Fig. 6b). In biofilm incubated with 500 µg/mL zinc oxide, it was noticed that nanoparticles were aggregated between the more prominent groups of cells (Fig. 5f). In contrast, in the case of 7.31 µg/mL of ZnO, no pronounced effects were noted. However, in a sample collected from the liquid culture, zinc oxide nanoparticles were well-dispersed on the surface of the cells (Fig. 6c). Presented results show that ZnO nanoparticles, given in the same concentration, are distributed differently in biofilm and the culture with planktonic forms. Perhaps, the aggregation of the nanoparticles allows reducing their anti-bacterial properties. Moreover, SEM analyses did not indicate pronounced toxicity of the nanomaterials. The integrity of some cells was not maintained; however, it can probably be attributed to the age of the culture or short dehydration time, as it was also observed in the control samples.
Obtained results show dependencies between different types of nanomaterials (applied in different concentrations) and the physiological response in P. aeruginosa culture. Interestingly, our outcome imposes that relatively low concentrations of ZnO nanoparticles generate higher pyocyanin and general fluorescence signals, simultaneously expressing lower signals from pyoverdine. Despite these observations, it did not influence the biofilm formation and only slightly inhibited the growth of P. aeruginosa. Based on the search in the Scopus database, this is the first report of the stimulative effect of ZnO or MWCNT on pyocyanin production. Most available research focused on inhibiting pyocyanin production due to its role in pathogenesis and tested only high concentrations of nanoparticles. Regarding the stimulative effect of high MWCNT concentrations, we hypothesize that P. aeruginosa cells may use the nanomaterial as a ‘scaffold’ that allows them to remain in the interphase, where oxygen, an essential regulator of the process, is abundant (Chincholkar and Thomashow 2013). In the light of these findings, further research, including genetic analyses and biofilm structure imaging, should be carried out to find the underlying mechanism of bacteria–nanomaterial interaction.
Conclusions
Obtained results provide the data showing that nanoparticles, i.e., MWCNT and ZnO, can be used as modulators of pyocyanin production. We have shown that ZnO nanoparticles applied in specific concentrations can change the profile of secondary metabolite production. As indicated in the culture conditions assay and considering other tests, including nanomaterials and AA, the preferred culturing method should be characterized with a low ratio of the thin layer of the culture to interphase. Optimal concentrations of nanomaterials for pyocyanin production established in this research are 7.31 µg/mL of ZnO, 500 µg/mL of MWCNT, and 125 µg/mL of MWCNT-AA. Our findings may be helpful in bioprocessing as pyocyanin remains a rather costly chemical substance.
References
Ali SG, Ansari MA, Alzohairy MA et al (2020) Effect of biosynthesized ZnO nanoparticles on multi-drug resistant Pseudomonas aeruginosa. Antibiot (basel, Switzerland) 9:260. https://doi.org/10.3390/antibiotics9050260
Augustyniak A, Cendrowski K, Grygorcewicz B et al (2020) The response of Pseudomonas aeruginosa pao1 to uv-activated titanium dioxide/silica nanotubes. Int J Mol Sci 21:1–15. https://doi.org/10.3390/ijms21207748
Augustyniak A, Jablonska J, Cendrowski K et al (2021) Investigating the release of ZnO nanoparticles from cement mortars on microbiological models. Appl Nanosci. https://doi.org/10.1007/s13204-021-01695-w
Bacame-Valenzuela FJ, Pérez-García JA, Castañeda-Zaldivar F, Reyes-Vidal MY (2020) Pyocyanin biosynthesis by Pseudomonas aeruginosa using a biodiesel byproduct. Mex J Biotechnol 5:1–16. https://doi.org/10.29267/MXJB.2020.5.3.1
Cabeen MT (2014) Stationary phase-specific virulence factor overproduction by a lasR mutant of Pseudomonas aeruginosa. PLoS One. https://doi.org/10.1371/journal.pone.0088743
Chen M, Sun Y, Liang J et al (2019) Understanding the influence of carbon nanomaterials on microbial communities. Environ Int 126:690–698. https://doi.org/10.1016/j.envint.2019.02.005
Chen JP, Li X-CC (2004) Organic light-emitting device having phenanthroline-fused phenazine
Chincholkar S, Thomashow L (2013) Microbial phenazines. Springer, Berlin
da Silva AJ, CunhaHreha JST et al (2021) Metabolic engineering of E. coli for pyocyanin production. Metab Eng 64:15–25. https://doi.org/10.1016/j.ymben.2021.01.002
Debritto S, Gaj TD, Satapute P, Sundaram L (2020) Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci Rep. https://doi.org/10.1038/s41598-020-58335-6
Dieppois G, Ducret V, Caille O, Perron K (2012) The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in pseudomonas aeruginosa. PLoS One. https://doi.org/10.1371/journal.pone.0038148
Elbargisy RM (2021) Optimization of nutritional and environmental conditions for pyocyanin production by urine isolates of Pseudomonas aeruginosa. Saudi J Biol Sci 28:993–1000. https://doi.org/10.1016/j.sjbs.2020.11.031
El-Deeb NM, Abo-Eleneen MA, Al-Madboly LA et al (2020) Biogenically synthesized polysaccharides-capped silver nanoparticles: immunomodulatory and antibacterial potentialities against resistant Pseudomonas aeruginosa. Front Bioeng Biotechnol 8:1–18. https://doi.org/10.3389/fbioe.2020.00643
El-Fouly MZ, Sharaf AM, Shahin AAM et al (2015) Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci 8:36–48. https://doi.org/10.1016/j.jrras.2014.10.007
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthase and evolutionary implications. J Bacteriol 172:884–900. https://doi.org/10.1128/jb.172.2.884-900.1990
Grygorcewicz B, Roszak M, Golec P et al (2020) Antibiotics Act with vB_AbaP_AGC01 Phage against Acinetobacter baumannii in human heat-inactivated plasma blood and Galleria mellonella models. Int J Mol Sci 21:4390
Gudkov SV, Burmistrov DE, Serov DA et al (2021) A mini review of antibacterial properties of ZnO nanoparticles. Front Phys 9:49
Hall S, McDermott C, Anoopkumar-Dukie S et al (2016) Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins (basel) 8:1–14. https://doi.org/10.3390/toxins8080236
Jancheva M, Thomas B (2021) A metabolite of pseudomonas triggers prophage-selective lysogenic to lytic conversion in Staphylococcus aureus. J Am Chem Soc. https://doi.org/10.1021/jacs.1c01275
Jurado-Martín I, Sainz-Mejías M, McClean S (2021) Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci 22:1–37. https://doi.org/10.3390/ijms22063128
Kavosi A, Hosseini Ghale Noei S, Madani S et al (2018) The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-26790-x
Kohatsu H, Kamo S, Furuta M et al (2020) Synthesis and cytotoxic evaluation of N-alkyl-2-halophenazin-1-ones. ACS Omega 5:27667–27674. https://doi.org/10.1021/acsomega.0c04253
Kolahalam LA, Kasi Viswanath IV, Diwakar BS et al (2019) Review on nanomaterials: synthesis and applications. Mater Today Proc 18:2182–2190. https://doi.org/10.1016/j.matpr.2019.07.371
Kovach K, Sabaraya IV, Patel P et al (2020) Suspended multi-walled, acid-functionalized carbon nanotubes promote aggregation of the opportunistic pathogen Pseudomonas aeruginosa. PLoS One 15:1–13. https://doi.org/10.1371/journal.pone.0236599
Lah NAC, Zubir MNM, Samykano MA (2018) Chapter 20—engineered nanomaterial in electronics and electrical industries. In: Mustansar Hussain CBT-H of N for IA (ed) Micro and Nano Technologies. Elsevier, pp 324–364
Laverty G, Gorman SP, Gilmore BF (2014) Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens 3:596–632. https://doi.org/10.3390/pathogens3030596
Lee JH, Kim YG, Cho MH, Lee J (2014) ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res 169:888–896. https://doi.org/10.1016/j.micres.2014.05.005
Limoli DH, Whitfield GB, Kitao T et al (2017) Pseudomonas aeruginosa alginate overproduction promotes co-existence with staphylococcus aureus in a model of cystic fibrosis respiratory infection. Mbio 8:1–18
Ma P-C, Siddiqui NA, Marom G, Kim J-K (2010) Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos Part A Appl Sci Manuf 41:1345–1367. https://doi.org/10.1016/j.compositesa.2010.07.003
Markowska-Szczupak A, Endo-Kimura M, Paszkiewicz O, Kowalska E (2020) Are titania photocatalysts and titanium implants safe? Review on the toxicity of titanium compounds. Nanomater 10
Meena M, Swapnil P, Divyanshu K et al (2020a) PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives. J Basic Microbiol 60:828–861. https://doi.org/10.1002/jobm.202000370
Meena S, Vidya Kalaivani M, Tripathi AD, Ramyaa Lakshmi T (2020b) Optimization and characterization of Alginic acid synthesized from a novel strain of Pseudomonas stutzeri. Biotechnol Rep 27:e00517. https://doi.org/10.1016/j.btre.2020.e00517
Mishra PK, Mishra H, Ekielski A et al (2017) Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today 22:1825–1834. https://doi.org/10.1016/j.drudis.2017.08.006
Moayedi A, Nowroozi J, Akhavan Sepahy A (2018) Cytotoxic effect of pyocyanin on human pancreatic cancer cell line (Panc-1). Iran J Basic Med Sci 21:794–799. https://doi.org/10.22038/ijbms.2018.27865.6799
Mohanty A, Wei L, Lu L et al (2015) Impact of sublethal levels of single-wall carbon nanotubes on pyoverdine production in Pseudomonas aeruginosa and its environmental implications. Environ Sci Technol Lett 2:105–111. https://doi.org/10.1021/acs.estlett.5b00057
Myers JA, Curtis BS, Curtis WR (2013) Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys 6:4. https://doi.org/10.1186/2046-1682-6-4
Najafi M, Nakhaei Moghaddam M, Yousefi E (2021) The effect of silver nanoparticles on pyocyanin production of Pseudomonas aeruginosa isolated from clinical specimens. Avicenna J Med Biotechnol 13:98–103. https://doi.org/10.18502/ajmb.v13i2.5529
Ouyang K, Mortimer M, Holden PA et al (2020) Towards a better understanding of Pseudomonas putida biofilm formation in the presence of ZnO nanoparticles (NPs): Role of NP concentration. Environ Int. https://doi.org/10.1016/j.envint.2020.105485
Ozdal M (2019) A new strategy for the efficient production of pyocyanin, a versatile pigment, in Pseudomonas aeruginosa OG1 via toluene addition. 3 Biotech 9:1–8. https://doi.org/10.1007/s13205-019-1907-1
Ozdal M, Gurkok S, Ozdal OG, Kurbanoglu EB (2019) Enhancement of pyocyanin production by Pseudomonas aeruginosa via the addition of n-hexane as an oxygen vector. Biocatal Agric Biotechnol 22:101365. https://doi.org/10.1016/j.bcab.2019.101365
Peruzzo R, Corrà S, Costa R et al (2021) Exploiting pyocyanin to treat mitochondrial disease due to respiratory complex III dysfunction. Nat Commun 12:1–19. https://doi.org/10.1038/s41467-021-22062-x
Saleh MM, Sadeq RA, Abdel Latif HK et al (2019) Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. Afr Health Sci 19:2043–2055. https://doi.org/10.4314/ahs.v19i2.28
Shen HB, Yong XY, Chen YL et al (2014) Enhanced bioelectricity generation by improving pyocyanin production and membrane permeability through sophorolipid addition in Pseudomonas aeruginosa-inoculated microbial fuel cells. Bioresour Technol 167:490–494. https://doi.org/10.1016/j.biortech.2014.05.093
Siddiqi KS, ur Rahman A, Tajuddin, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. https://doi.org/10.1186/s11671-018-2532-3
Sikora P, Augustyniak A, Cendrowski K et al (2016) Characterization of mechanical and bactericidal properties of cement mortars containing waste glass aggregate and nanomaterials. Materials (basel) 9:701. https://doi.org/10.3390/ma9080701
Sikora P, Augustyniak A, Cendrowski K et al (2018) Antimicrobial activity of Al2O3, CuO, Fe3O4, and ZnO nanoparticles in scope of their further application in cement-based building materials. Nanomaterials 8:1–15. https://doi.org/10.3390/nano8040212
Sismaet HJ, Webster TA, Goluch ED (2014) Up-regulating pyocyanin production by amino acid addition for early electrochemical identification of Pseudomonas aeruginosa. Analyst 139:4241–4246. https://doi.org/10.1039/c4an00756e
Slininger PJ, Jackson MA (1992) Nutritional factors regulating growth and accumulation of phenazine 1-carboxylic acid by Pseudomonas fluorescens 2–79. Appl Microbiol Biotechnol 37:388–392. https://doi.org/10.1007/BF00210998
Soler-Arango J, Figoli C, Muraca G et al (2019) The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: a comprehensive model explaining plasma-mediated biofilm eradication. PLoS One 14:1–27. https://doi.org/10.1371/journal.pone.0216817
Tiwari V, Mishra N, Gadani K et al (2018) Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front Microbiol 9:1218
Viana AAG, de Oliveira BTM, Cavalcanti TG et al (2018) Correlation between pyocyanin production and hydrocarbonoclastic activity in nine strains of Pseudomonas aeruginosa. Int J Adv Eng Res Sci 5:212–223. https://doi.org/10.22161/ijaers.5.7.28
Wang Y, Mortimer M, Chang CH, Holden PA (2018) Alginic acid-aided dispersion of carbon nanotubes, graphene, and boron nitride nanomaterials for microbial toxicity testing. Nanomaterials. https://doi.org/10.3390/nano8020076
Yang Y, Alvarez PJJ (2015) Sublethal concentrations of silver nanoparticles stimulate biofilm development. Environ Sci Technol Lett 2:221–226. https://doi.org/10.1021/acs.estlett.5b00159
Yu D, Yong Y-C, Liu C et al (2017) New applications of genetically modified Pseudomonas aeruginosa for toxicity detection in water. Chemosphere 184:106–111. https://doi.org/10.1016/j.chemosphere.2017.05.154
Zanni E, Bruni E, Chandraiahgari CR et al (2017) Evaluation of the anti-bacterial power and biocompatibility of zinc oxide nanorods decorated graphene nanoplatelets: new perspectives for antibiodeteriorative approaches. J Nanobiotechnol 15:1–12. https://doi.org/10.1186/s12951-017-0291-4
Zhao J, Wu Y, Alfred AT et al (2014) Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett Appl Microbiol 58:541–548. https://doi.org/10.1111/lam.12224
Acknowledgements
This research was funded by the National Science Center (Poland) within project No. 2018/31/N/NZ1/03064 (PRELUDIUM 16). Adrian Augustyniak was supported by the German Research Foundation (DFG) as part of the Research Training Group on Urban Water Interfaces (GRK 2032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Supplementary information
Supplementary materials are available and contain Materials and methods, and Results sections.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Submitted to special issue NANO 2021 within the 9th International Conference “Nanotechnologies and Nanomaterials” NANO-2021.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jabłońska, J., Dubrowska, K., Augustyniak, A. et al. The influence of nanomaterials on pyocyanin production by Pseudomonas aeruginosa. Appl Nanosci 12, 1929–1940 (2022). https://doi.org/10.1007/s13204-022-02461-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02461-2