Abstract
CdO thin films were prepared by electrodeposition method. Solution temperatures were varied from 58 to 98 °C. It was understood from the film thicknesses and current densities that the reaction rate increased as the temperature increased. Good crystallization and a thick film were obtained at 98 °C. It was found that the energy band gap varied between 1.99 and 2.61 eV depending on the bath temperature. SEM images also showed that surface morphologies were dependent on bath temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Science and technology have given rise to a tremendous escalation in this century. In the fields of science and technology, nanotechnology is much substantial. Nano-sized research has opened revolutionary opportunities for a wide number of technological applications in the past decade (Kalpanadevi et al. 2013). Regarding the nano size materials, transparent metal oxide films are of technological importance for liquid crystal displays, chemical sensors for solar cells, and nanoporous films for dye sensitized solar cells (Jayakrishnan and Hodes 2003). Among different metal oxides, CdO is an important n-type semiconductor metal oxide, which belongs to the II–VI group (Giribabu et al. 2013). CdO has a large direct band gap of 2.2 eV (Baykul and Orhan 2014) which is a promising candidate for optoelectronic applications such as solar cells, phototransistors, gas sensors, photodiodes, and transparent electrodes (Fan 2009).
At present, CdO has been prepared by a large range of techniques including sol–gel, sputter deposition, spray pyrolysis, MOCVD, molecular beam epitaxy, activated reactive evaporation (Liu et al. 2012), and electrodeposition. Electrodeposition is a significant method that is used for the growth of metal oxide films from aqueous solutions and when compared with other techniques the advantages of this method include inexpensiveness, low process temperature, and opportunity to control the morphology of the films (Singh et al. 2011a).
When Cd(NO3)2 was used in the deposition, the following reaction took place (Singh et al. 2011a),
After the deposition, if Cd(OH)2 was heated at a relatively high temperature, it converted to CdO (Singh et al. 2011b),
So far, CdO thin films were deposited in aqueous solutions at various bath temperatures such as 30 °C (Singh et al. 2011a), 35 °C (Abdulridha 2016), 70 °C (Baykul and Orhan 2014; Singh et al. 2011b), 80 °C (Singh et al. 2011b), and 90 °C (Liu et al. 2012; Singh et al. 2011b). Among these studies, there was only one study that the effects of bath temperature on CdO films were investigated at 70, 80, and 90 °C (Singh et al. 2011b) but there was no any other research on wide range values of bath temperature.
In this work, the effects of bath temperature were investigated in detail. Bath temperatures were varied between 58 and 98 °C. It is understood from the XRD patterns that the good crystallization and thick film were formed at the bath temperature of 98 °C. Besides, this study showed that the film was not obtained at temperature below of 58 °C. Unlike other studies, it was found that crystallite size and energy band gap strongly depended on bath temperature.
Experimental procedure
In this study, polycrystalline of CdO was obtained by using electrodeposition device of IVIUM VERTEX. Three-electrode electrodeposition cell was employed for the deposition of Cd(OH)2 containing (indium doped tin oxide) ITO as a working electrode, platinum wire as a counter electrode, and Ag/AgCl as a reference electrode. ITO coated glass substrates were washed with acetone and water for 5 min to ensure that no pollution was left on them. Deposition baths consisted of 0.01 M Cd(NO3)2 and 0.1 M KCl as a supporting electrode and cathodic potentials were chosen to be −0.71 eV. Experimental conditions are given in Table 1. Bath temperatures were adjusted by a heater to 58 ± 1, 68 ± 1, 78 ± 1, 88 ± 1, and 98 ± 1 °C and were named T1, T2, T3, T4, and T5, respectively. When bath temperature was below of 58 °C, the film was not formed. Cd(OH)2 covered on ITO-coated glass substrates during the depositions and after then, samples were annealed in air for 1 h at 445 °C with an oven. The samples were left to cool in the oven after the annealed. Thus, the color of samples converted from gray to orange.
To investigate the crystalline quality, the crystallographic phase, and the possible texture of CdO thin films, PANalytical empyrean X-ray diffractometer using CuKα(λ = 1.54 Å) radiation in 2θ range 30°–70° was used. Scan rate is 2º/min. JASCO V–530 double-beam UV–Vis spectrophotometer was employed in order to measure absorbance values of the CdO films. Also a Zeiss supra 40VP scanning electron microscopy (SEM) was performed to investigate the morphology.
Result and discussion
Structural studies
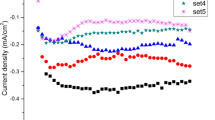
The gravimetric method was used to measure the thicknesses of the CdO films, and thicknesses are given in Table 2. As the temperature increased from 58 to 98 °C, the film thickness increased from 402 to 783 nm. These results were supported by current densities. Current densities for the films are given in Fig. 1. When the temperature was 58 °C, the current densities were averagely 0.05 mA/cm2. Besides, as the temperature increased from 58 to 98 °C, the current densities increased up to 1.1 mA/cm2 proportionally. Thus, it could be said that as the temperature increased, the reaction rate increased. It was concluded that when deposition temperature was increased, the molecular collision increased, therefore, the reaction rate increased.
The XRD patterns of the CdO films are shown in Fig. 2. It is also shown in Fig. 2 that the peak intensities increased as the temperature of depositions increased. There are two reasons for these results. One of these is good crystallization, and the other one is the increasing film thicknesses. Apart from ITO peaks, observed diffraction peaks (1 1 1), (0 0 2) (0 2 2), and (2 2 2) for CdO belong to the polycrystalline cubic structure of CdO.
By employing the Scherrer’s equation which is given in Eq. 4, the average grain size was calculated for CdO thin films
where β is the full width at the half maximum of peak height (in degrees), 2θ C is the position of peak center, and λ is the wavelength of X-ray radiation (1.54056 Å) (Bhowmik et al. 2008). Crystallite sizes of the films are given in Table 2. It is shown in Table 2 that crystallite sizes varied from 39 nm to 84 depending on increasing reaction rates. It is reported in a previous study (Kiyak Yildirim and Altiokka 2017) that when the reaction rate increased, the crystallite size increased, and thus band gap decreased. This study showed that crystallite size and reaction rate were affected strongly by temperature. It is concluded that bath temperature affects kinetic energy of ions and molecules. Therefore, collisions increase. Increased collisions increase the reaction rate. Increasing the reaction rate increases the crystal growth rate so increases in crystallite size.
Optical studies
It is a well-known method that measuring of absorbance is employed to calculate optical properties of the films. Figure 3 shows absorbance values of the CdO thin films. When deposition temperature was 58 °C, the absorbance was nearly 0.5. Besides when temperature increased up to 98 °C, the absorbance was nearly 1.5. It was concluded that film thicknesses caused these results. As a result, absorbance depends on film thicknesses, and film thicknesses also depend on temperature.
The Tauc plot was employed to estimate band gaps of the CdO films, and it is given in Eq. 5. The relationship between the absorption coefficient and photon energy for direct allowable transition can be denoted as (Vishakha et al. 2013; Mkawi et al. 2015);
where α is the absorption coefficient, A is a constant, and hv is the energy of a photon with 1240/λ. The linear parts of the curves were extended to the point of α = 0, and the intercept on the abscissa was the band gap (Eg) (Vishakha et al. 2013). The band gaps for the CdO thin film are given in Fig. 4. The band gaps of the CdO thin films were between 2.61 and 1.99 eV. It was interesting that band gap depended on bath temperatures because of the fact that bath temperature affected reaction rate and thus crystallite size. For the bulk matter, the bands are formed by the merger energy levels of atoms. When the crystallite size decreases, the number of overlapping of orbitals decreases and therefore valance and conduction bands get narrower. When bands get narrower, the gap between valence and conduction band gets larger. Therefore, band gap increases.
Morphology studies
Figure 5 shows the SEM images of the prepared CdO films magnified 20,000 times. Figure 5a shows SEM image of CdO thin film obtained at 58 °C. This surface consists of a lace-like structure; diameters of wires are averagely 100 nm, and lengths of wires are up to 2 µm. Figure 5b and c show the SEM image of the films obtained at 68 and 78 °C, respectively. These two images are nearly same. There are clusters of CdO crystal on the sheet-like structure. SEM images of the films obtained at 88 and 98 °C are given in Fig. 5d and e, respectively. There are platelets stacks on these surfaces and diameters of these platelets are averagely 750 nm. In the literature (Giribabu et al. 2013), these morphologies of the CdO are found. This study showed that surface morphology depends strongly on deposition temperature.
Conclusion
In this work, thin films of CdO samples were obtained by employing electrodeposition. Effects of deposition temperature were investigated in a wide range such as 58 and 98 °C for the first time. The current densities showed that when deposition temperature increased, reaction rate and film thicknesses increased. Structural analyzes were studied by using XRD, and XRD studies showed that when CdO film was produced at 98 °C, the film formed in good crystalline and thick. The optical properties of the CdO thin films were determined by using absorbance measurement. As the bath temperatures increased, absorbance also increased and band gap decreased from 2.61 to 1.99 eV. The images of surfaces were taken by using a SEM. SEM images showed that surface morphology depended on bath temperature. A lace-like structure was formed at relatively low temperature, and a platelet-like structure was formed at relatively high temperature.
References
Abdulridha WM (2016) Synthesis and study the structure and optical properties of CdO polycrystalline thin film using electrochemical depositing method. Int J Nanomater Chem 2:21–25. doi:10.18576/ijnc/020104
Baykul MC, Orhan N (2014) Effect of seed layer on surface morphological, structural and optical properties of CdO thin films fabricated by an electrochemical deposition technique. Solid State Electron 101:29–32. doi:10.1016/j.sse.2014.06.033
Bhowmik RN, Nrisimha Murty M, Sekhar Srinadhu E (2008) Magnetic modulation in mechanical alloyed Cr1.4Fe0.6O3 oxide. PMC Phys B 1:20–38. doi:10.1186/1754-0429-1-20
Fan DH (2009) Catalyst-free growth and crystal structures of CdO nanowires and nanotubes. J Cryst Growth 311:2300–2304. doi:10.1016/j.jcrysgro.2009.01.088
Giribabu K, Suresh R, Manigandan R, Stephen A, Narayanan V (2013) Cadmium oxide nanoplatelets: synthesis, characterization and their electrochemical sensing property of catechol. J Iran Chem Soc 10:771–776. doi:10.1007/s13738-012-0211-3
Jayakrishnan R, Hodes G (2003) Non-aqueous electrodeposition of ZnO and CdO films. Thin Solid Films 440:19–25. doi:10.1016/S0040-6090(03)00811-3
Kalpanadevi K, Sinduja CR, Manimekalai R (2013) Characterisation of zinc oxide and cadmium oxide nanostructures obtained from the low temperature thermal decomposition of inorganic precursors. ISRN Inorg Chem. doi:10.1155/2013/823040
Kiyak Yildirim A, Altiokka B (2017) Effects of concentration on CdO films grown by electrodeposition. Appl Nanosci 7:131–135. doi:10.1007/s13204-017-0552-4
Liu ZQ, Guo R, Li GR, Bu Q, Tong YX (2012) Facile electrodeposition of large-area CdO and Cd1–xCoxO curved nanowires. Electrochim Acta 59:449–454. doi:10.1016/j.electacta.2011.10.095
Mkawi EM, Ibrahim K, Ali MKM, Farrukh MA, Mohamed AS (2015) The effect of dopant concentration on properties of transparent conducting Al-doped ZnO thin films for efficient Cu2ZnSnS4 thin-film solar cells prepared by electrodeposition method. Appl Nanosci 5:993–1001. doi:10.1007/s13204-015-0400-3
Singh T, Dk Pandya, Singh R (2011a) Annealing studies on the structural and optical properties of electrodeposited CdO thin films. Mater Chem Phys 130:1366–1371. doi:10.1016/j.matchemphys.2011.09.035
Singh T, Pandya DK, Singh R (2011b) Electrochemical deposition and characterization of elongated CdO nanostructures. Mater Sci Eng, B 176:945–949. doi:10.1016/j.mseb.2011.05.021
Vishakha N, Dhanwate N, Chaure B (2013) Effect of growth potential on the electrodeposition of CIS thin films. Appl Nanosci 3:1–5. doi:10.1007/s13204-012-0063-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kıyak Yıldırım, A., Altıokka, B. An investigation of effects of bath temperature on CdO films prepared by electrodeposition. Appl Nanosci 7, 513–518 (2017). https://doi.org/10.1007/s13204-017-0591-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0591-x