Abstract
Thin films of copper indium diselenide (CIS) were prepared in aqueous bath by low-cost potentiostatic electrodeposition technique onto Fluorine doped tin oxide substrates. The deposition potential was optimized using cyclic voltammetry study in a ternary Cu–In–Se system. The films were characterized systematically with the aid of UV–Vis spectroscopy, IV measurements, X-ray diffraction, Scanning electron microscopy and Energy dispersive X-ray analysis. CIS films deposited for different growth potential shows the tetragonal structure with (112) preferential orientation. Annealing of the films at temperature 400°C not only improves the crystallinity of layers, but it also increases the grain size, which is suitable for the development of high efficiency solar cells.
Similar content being viewed by others
Introduction
Ternary chalcopyrite compounds CuInX2 (X = S, Se, Te) are being considered as important semiconductor materials for thin film solar cell applications because it has a direct band gap (1.08 eV) which can be tailored by the addition of gallium, high optical-absorption coefficient, good stability, largest efficiency, cost effective and chemically and environmentally stable (Arya et al. 1991; Chaure et al. 2005). Furthermore, these compounds can be easily deposited either n- or p-type thin film form by controlling the elemental composition (Chaure et al. 2004; Chraibi et al. 2004). A CIGS solar cell achieved the highest efficiency of over 20% on laboratory scale with high-vacuum techniques (Repins et al. 2008) which indicate that CIS-based solar cells are able to compete with Si-based solar cell. Various other deposition techniques apart from electrodeposition are used by researcher such as sputtering (Yamaguchi et al. 1992), co-sputtering of the elements (Arya et al. 1991), Rapid thermal processing (Karg et al. 1993), Pulse laser deposition (Wang et al. 2010), Spray pyrolysis (Bates et al. 1983) and Molecular beam epitaxy (Niki et al. 2002), etc.
In this paper we report the cathodic electrodeposition of CuInSe2 on a Fluorine doped tin oxide (FTO) substrates. Advantages of electrodeposition are low equipment cost, high deposition rate, negligible waste of chemicals and possibility to deposit over large area. Moreover, an isothermal process mainly controlled by concentration, temperature, pH of the electrolyte and applied potential. A Pourbaix diagram has been used as a guide to fix the preliminary electrodeposition conditions, pH and temperature of the electrolyte, concentration of ionic species etc. Thin film deposition under various conditions have been performed to understand the deposition mechanism and to optimize the deposition condition. The elemental composition and structural properties of the CIS thin films were studied by using Energy dispersive X-rays analysis and the X-ray diffraction technique.
Experimental details
CuInSe2 (CIS) thin films were deposited on FTO-coated glass substrates of sheet resistance 15–20 Ω/cm2. A conventional three-electrode electrochemical cell was employed for the deposition of thin films consisting FTO as a working electrode, graphite as a counter electrode and Ag/AgCl as a reference electrode (Pandey et al. 1996). The ionic source for Cu, In and Se was CuSO4 ·5H2O, In2(SO4)3·5H2O and H2SeO3. The details are reported elsewhere (Chaure et al. 2004). Lithium sulfate was used as a supporting electrolyte, to bring deposition potential of all species closer for a better co-deposition of Cu, In and Se. Triethanolamine (TEA) was used as a complexing agent which forms complex with copper that reduces the rate of copper reduction and shifts copper deposition potential towards cathodic direction. Triethanolamine also improves the adhesion of film to the substrate. Similar results are reported by Whang et al. (2009) and Yu et al. (2010). Indeed, we found that the films deposited in presence of TEA were highly uniform, very well adhesive to the substrate and free of loosely bound particles on the surface of sample. The above precursors were dissolved in double-distilled water with appropriate molar concentration. The pH of bath was adjusted ~2 using H2SO4 or NaOH. Cyclic voltammetry (CV) and potentiostatic deposition of CIS layers were carried out using μ3AUT 70762 AUTOLAB potentiostat of working temperature ~40°C without stirring. The information observed in CV was used to optimize the film deposition potential. The films were deposited at various growth potentials ranging from −0.6 to −0.9 V with respect to Ag/AgCl. The layers were annealed ~400°C for 10 min to improve homogeneity and crystalline nature. Subsequently, the films were characterized using range of characterization techniques.
Results and discussion
Cyclic voltammetry was recorded to understand the co-deposition procedure of Cu, In and Se. Figure 1 shows the CV recorded for CIS bath on FTO working electrode. The features related to the deposition of elemental, binary and ternary phases of CIS were observed. Initially, at lower cathodic potential up to −0.4 V, the features noticed were related to the reduction of Cu and Se by charge-transfer reaction;
The deposition of indium was observed beyond the potential −0.6 V with respect to Ag/AgCl reference electrode, by following charge-transfer reaction;
A plateau observed in between −0.7 and −0.9 V favors the deposition of CIS alloys. Sharp linear rise in cathodic current observed beyond −0.9 V could be due to the metallic deposition of indium along with hydrogen evolution. CIS can be formed by the chemical reaction:
During anodic curve, the dissolution (stripping) peaks for In, Cu and Se were observed and marked as a, b and c, respectively.
Theoretical thickness of the films deposited at various potentials was calculated using Faraday’s law:
where ‘n’ is the number of electron transferred, ‘F’ is the Faraday’s constant, ‘A’ is the area of electrode, ‘i’ is the current, ‘t’ is the deposition time, ‘M’ is the formula weight, and ‘ρ’ is the density of intrinsic CIS (Friedfeld et al. 1999).
Thickness measured for the samples deposited at potential −0.8 and −0.9 V was 590 and 650 nm (±20 nm), respectively. Note that the deposition time was kept constant (10 min) for the deposition of above samples.
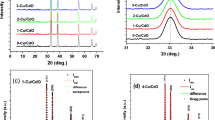
Structural characterization was carried out with the help of X-ray diffraction (D8 Bruker). The X-ray diffractogram of the CIS thin film deposited at different potential is shown in Fig. 2. The presence of sharp peaks in the X-ray diffractogram shows that CIS is polycrystalline in nature. The CIS is found to exhibit chalcopyrite tetragonal structure with a preferred orientation along certain crystallographic planes which have been identified as (112), (204/220), (116/312). The secondary binary phases Cu x Se and In x Se were observed for the film deposited for −0.6, −0.7 and −0.9 V, whereas stoichiometric film along with high intensity CIS peaks were observed for the layer deposited at −0.8 V. Note that no secondary phases were observed for film deposited at −0.8 V. The substrates (FTO) peaks were also observed in XRD spectra which are marked as solid circles. Lattice parameter for tetragonal structure can be obtained by the formula,
The lattice parameter was calculated to be 5.783 and 11.73 Å which gives tetragonal distortion i.e., c/a ratio is approximately equal to 2.
Post deposition heat treatment on CIS films was carried out in the furnace at 400°C (± 5°C) for 10 min. After heat treatment the peak intensity improves which indicates the improvement in crystallinity of CIS thin films (Kemell Marianna et al. 2000). The X-ray patterns obtained for as-deposited and annealed CIS thin film are shown in (Fig. 3).
The grain size of as-deposited and annealed sample was calculated using the Scherrer equation,
where λ is the wavelength, β is the FWHM and θ is the diffraction angle (Cullity and Stock 1978). Grain size of the as-deposited and annealed sample of CIS was calculated approximately 13 and 24 nm, respectively (Panchal et al. 2009).
Compositional and morphological analysis was carried out using SEM and EDAX (JEOL JSM-6360A). Figure 4a–d shows the SEM images of as-deposited CIS, deposited at potential −0.6, −0.7, −0.8 and −0.9 V, respectively. All the films show uniform and compact deposition. CIS layer deposited at −0.8 V was compact, spherical and uniform, whereas the mixed morphology consisting uniform grains along with irregular shaped clusters were observed for other samples. After annealing CIS films become compact and power free with grayish metallic finishing. Heat treated sample (Fig. 5) can be clearly seen the grain enhancement along with noticeable improvement in crystallinity.
The elemental composition was detected by EDAX at accelerated voltage 20 kV and probe current 1nA. Table 1 shows the chemical composition of as deposited CIS films at potentials −0.6, −0.7, −0.8 and −0.9 V.
CIS layer deposited at −0.8 V was nearly stoichiometric ~ 24:25:51 for Cu:In:Se. The growth potential plays an important role to control the stoichiometry of layer. The Cu-rich and In-rich layers are p-type and n-type, respectively (Chaure et al. 2005). The optical band gap was estimated from Fig. 6 by plotting the graph (αhν)2 as a function of (hν). The electronic transition between the valance and conduction band in case of CIS was direct transition. The transition of electron can be allowed as permitted by the transition probability or forbidden where no such probability exists. The transition probability is given by the Eq. 8. The absorption coefficient was calculated of the order of 104 per cm. Similar value is reported by Friedfeld et al. (1999).
where Eg denotes the band gap, hν the energy of the incident photon and α0 is a constant. Value of Eg was obtained by extrapolating the linear portion of the graph to intercept the photon energy axis. After annealing, the band gap comes to very closer to that of ideal value which is 1.04 eV.
Electrical measurements
The electrical studies were performed on FTO/CIS/Al structure (Fig. 7).
An electrical measurement shows Schottky behavior of diode. IV curve clearly shows that the improvement in conductivity after heat treatment. The improvement in current indicates the decrease in grain boundaries and defects.
Conclusion
CIS films were potentiostatically electrodeposited from aqueous bath. A plateau observed between −0.7 and −0.9 V was found to be suitable for the deposition of CIS films. CIS films deposited at reported potentials were polycrystalline with tetragonal chalcopyrite structure. The optical band gap was estimated to be 1.04 eV. SEM study showed that the CIS films are uniform, densely compact, and free from pores. From EDAX, it has been observed that the growth potential plays an important role to control the stoichiometry of film. Stoichiometry of the thin film deposited at −0.8 V is very close to the desired composition of film. The current density has been increased for heat treated sample could be due to change in conductivity of material as well as the defects present in as-deposited sample may be reduced. Further study on CIS is under progress and the development of superstrate and substrate solar cell devices will be the topic of future work.
References
Arya RR, Lommasson T, Fieselmannm B, Russell L, Carr L, Catalano A (1991) CIS solar cells by elemental sputtering. Rec IEEE Photovolt Special Conf 2:903–906
Bates CW, Uekita M, Nelson KF, Abernathy CR, Mooney JB (1983) Effect of pH on the production of chalcopyrite CuInSe2 prepared by spray pyrolysis. Appl Phys Lett 43:851–852
Chaure NB, Young J, Samantilleke AP, Dharmadasa IM (2004) Electrodeposition of p–i–n type CuInSe2 multilayers for photovoltaic. Appl Solar Energy Mater Solar Cells 81:125–133
Chaure NB, Samantilleke AP, Burton RP, Young J, Dharmadasa IM (2005) Electrodeposition of p+, p, i, n and n+-type copper indium gallium diselenide for development of multilayer thin film solar cells. Thin Solid Films Film 472:212–216
Chraibi F, Fahoume M, Ennaui A, Delplancke JL (2004) One step electrodeposition of CuInSe2 thin Films. M J Condensed Matter 5:1
Cullity BD, Stock SR (1978) Elements of X-ray diffraction. Prentie-Hall Inc, New Jersey
Friedfeld R, Raffaelle RP, Mantovani JG (1999) Electrodeposition of CuInxGa1~xSe2 thin films. Sol Energy Mater Sol Cells 58:375–385
Karg VP, Harms H, Rimmasch J, Riedl W, Kotschy J, Holz J, Treichler R, Eibl O, Mitwalskotschy A, Holz J, Kiendl A (1993) Novel rapid thermal processing for CIS thin film solar cell. Rec IEEE Photovol Special Conf 23:441–446
Marianna K, Ritala M, Leskela M (2000) Effects of post-deposition treatments on the photoactivity of CuInSe2 thin films deposited by the induced co-deposition mechanism. J Mater Chem 11:668–672
Niki S, Yamada A, Hunger R, Fons PJ, Iwata K, Matsubara K, Nishio A, Nakanishi H (2002) Molecular beam epitaxial growth and characterization of CuInSe2 and CuGaSe2 for device applications. J Cryst Growth 237–239:1993–1999
Panchal CJ, Rehani B (2009) Structural, optical, and electrical properties of flash-evaporated copper indium diselenide thin films. J Mater Sci 44:316–322
Pandey RK, Sahu SN, Chandra S (1996) Handbook of semiconductor electrodeposition. Marcel Dekker Inc., New York
Repins I, Contreras MA, Egaas B, DeHart C, Scharf J, Perkins CL, To B, Noufi R (2008) 19 9%-efficient ZnO/CdS/CuInGaSe2 solar cell with 81 2% fill factor. Prog Photovolt Res Appl 16:235–239
Wang XL, Wang GJ, Tian BL, Wan SM, Du ZL (2010) CuInSe2 thin films obtained by pulse-plating electrodeposition technique with novel pulse wave. Chin Sci Bull 55:1854–1858
Whang T-J, Hsieh M-T, Kao Y-C, Lee S-J (2009) A study of electrodeposition of CuInSe2 thin films with triethanolamine as the complexing agent. Appl Surf Sci 255:4600–4605
Yamaguchi T, Matsufusa J, Yoshida A (1992) Structural properties of CuInxGa1−xSe2 thin films prepared by rf sputtering. J Appl Phys 72:5657
Yu R, Ren T, Li C (2010) Effect of triethanolamine and sodium dodecyl sulfate on the formation of CuInSe2 thin films by electrodeposition. Thin Solid Films 518:5515–5519
Acknowledgments
The authors gratefully acknowledge the financial support received from Board of College and University Development (BCUD), University of Pune.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dhanwate, V.N., Chaure, N.B. Effect of growth potential on the electrodeposition of CIS thin films. Appl Nanosci 3, 1–5 (2013). https://doi.org/10.1007/s13204-012-0063-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0063-2