Abstract
Molecular simulations were performed to investigate the structural and electronic properties of graphene (G) nanosheet interacting with the monomer of chitosan (MCh) (C6H13O5N). The G nanosheet with the C54H18 chemical composition is modeled according to the armchair edge and is functionalized with boron atoms. The interaction between the nanosheet and the MCh is investigated to search for better bio-sensing characteristics. Simulations are done within the density functional theory, the generalized gradient approximation is applied to deal with the exchange–correlation energies, and the all-electron basis set with double polarization is used. To determine the structure stability, the minimum energy criterion is applied for the G + MCh system in seven different geometries; in addition, it is checked with the non-complex vibration frequency. Results show chemical interactions between the G nanosheets and the MCh in the ground-state geometry. In this geometry, the monomer is oriented perpendicular to the G nanosheet at a distance of 3.9 Å with the nanosheet remaining unchanged. The nanosheet functionalization with boron (to form an epoxy group) and interaction with the monomer yield improved adsorption conditions with a bond length of Cmesh–B–NAmine = 3.19 Å and the formation of B–N (boron attached to graphene–amine of the monomer) bond of length 1.57 Å. The polarity of the G + B and G + B + MCh systems displays ionic characteristics contrary to G behavior. The (HOMO–LUMO) energy difference is 1.30 eV for the G + B system and 0.75 eV for the G + B + MCh. Finally, the G + B + MCh system is investigated when D-(+)-glucose and cholesterol are adsorbed. Results show chemisorptions, which suggest the system to be used in biosensor devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The functionalization of graphene (G) surface (Novoselov et al. 2004) has given rise to the investigation of new materials such as graphane (Sofo et al. 2007; Elias et al. 2009), graphene oxide (GO) (Dikin et al. 2007; Li et al. 2009), graphone (Zhou et al. 2009), graphanol (Wang and Kaxiras 2010), fluorographene (FG) (Nair et al. 2010), bromination of graphene (Yaya et al. 2011), hydrographene (HG) (Ezawa 2011), chlorination of graphene (Li et al. 2011), GraPOSS (graphene + polyhedral oligomeric silsesquioxanes) (Valentini et al. 2011) and GO-POSS (Xue et al. 2012). In addition, it has motivated the surface modifications of graphene with polymers or other functional groups to vary the properties and find new future applications in optoelectronic devices and/or medicine. Chitosan is an abundant linear biopolymer; it occupies the third place in abundance as compared with cellulose and chitin. Moreover, it displays excellent characteristics to form membranes. Chitosan has good adhesion, excellent mechanical properties, good biocompatibility and good susceptibility for chemical modifications, as it contains a large amount of OH groups and amine functional groups (Kurita 2001; Masuko et al. 2005). One motivation to explore the graphene–chitosan system is the possibility of recovering materials from sea waste as shrimp skeleton and then to obtain chitosan (Hernández Cocoletzi et al. 2009).
Studies have been recently reported by Chigo Anota et al. (2013a) on the hexagonal boron nitride nanosheet functionalized with chitosan (hBN + MCh). Results show chemical interactions (chemisorption) with the ground-state geometry exhibiting a parallel configuration. When defects are generated in the mesh (double vacancy) of the hBN surface, the adsorption is slightly increased. Moreover in the interactions of the monomer with the BN nanotube of different chirality, the monomer is chemically adsorbed (Rodriguez Juárez et al. 2013). The adsorption process on the surface is improved with the presence of vacancies in the tubular systems.
On the other hand, Kang et al. (2009) have studied the electronic properties of the graphene–chitosan compound system. Results suggest that the system may be applied to glucose biosensor devices. These nanosheets have been manufactured using the McAllister et al. (2007) and Schniepp et al. (2006) methods with sheets being suitable for forming the composites. In this work it is invoked the model used by Chigo Anota et al. (2012, 2013b, 2013c) to represent the graphene or boron nitride nanosheet as a CnHm-like cluster to study the adsorption of the monomer of chitosan, represented by the monomeric unit (C6H13O5N), on the graphene surface (G + MCh). As another case, the adsorption of MCh on the G nanosheet functionalized with boron (G + B) is explored. The structure contains on one side of the central hexagon of the G nanosheet the functional group epoxy and a new B–N bond with the amine group of the monomer. In this way, the binding energy is increased and suggests the possible use of the system as a biosensor device. Moreover, the system has been tested as an adsorbent of glucose and cholesterol biomolecules. In this work, we report changes in the structural properties before and after the adsorption process of the polysaccharides: dipolar moment, chemical reactivity (chemical potential), adsorption energy and the energy difference of HOMO–LUMO frontier orbitals.
Calculation models and method
First principles total energy calculations are performed to study the following systems: graphene–chitosan (G + MCh), graphene doped with boron–chitosan (G + B + MCh), graphene doped with boron–chitosan D-(+)–glucose (G + B + MCh + glucose) and graphene doped with boron–chitosan–cholesterol (G + B + MCh + cholesterol). The exchange-correlation energies are treated with the Hamprecht–Cohen–Tozer–Handy (HCTH) (Boese and Handy 2001) functionals within the generalized gradient approximation (GGA) and the basis set DNP with double polarization (Delley 1990) (that is, p-orbitals of H and d-orbitals of boron, carbon, nitrogen and oxygen are considered) is applied as implemented in the quantum chemistry software DMol3 code (Delley 1990, 2000). The choice of the HCTH functional for the calculations is done because this allows describing adequately the interaction between the CnHm-like fragment and the graphene sheet as reported by Araujo et al. (2012).
The armchair nanosheets are of height 1.41 nm and of width 1.34 nm; they are mono-hydrogenated at edges (passivated). In this way, the CnHm-like clusters form a mesh with a total of 72 atoms (in 19 hexagons). The chitosan is modeled with the monomeric unity having the chemical composition C6H13O5N with 25 atoms. A total of seven configurations for the G + MCh system were explored: three geometries oriented to the chitosan OH groups, one oriented to the oxygen of the piranosic cycle, one perpendicular to the amine group and two parallel geometries to the carbon mesh. In all cases, charge neutrality was considered. The orbital cut radius is 0.37 nm (for the monomer of chitosan and cholesterol) and 0.47 nm (for the G, G + MCh, G + B + MCh, G + B + MCh + glucose and G + B + MCh + cholesterol) on the base function with a tolerance of 1.0 × 10−6 Ha for the energy convergence. The structural stability is achieved following the minimum criterion and the non-complex vibration frequencies (Foresman et al. 1996).
To validate the models, the cohesion energy [Ecoh = [nE(C) + mE(H)−E(CnHm)/(n + m)] of naphthalene (C10H8), phirene (C16H10), coronene (C24H12) and the cluster C55H18 was calculated, obtaining a value of 3.82 a.u./atom for all systems.
To improve the bioadsorption of D-(+)-glucose and cholesterol, the graphene sheet is functionalized with boron at the epoxy-kind structure and then joined to the monomer of chitosan at the nitrogen atom of the amine group. In this work, we report the structural changes (prior and after adsorption), polarity (dipole moment magnitude) and chemical reactivity using the formula μ = (HOMO + LUMO)/2, energy gap (HOMO–LUMO frontier orbitals difference) and adsorption energy [E(ad)1 = E(graphene + MCh)−E(graphene)−E(MCh), E(ad)2 = E(graphene + B+MCh)−E(graphene)−E(B)−E(MCh), E(ad)3 = E(graphene + B + MCh + X)−E(graphene)−E(B)−E(MCh)−E(X); X = D-(+)-glucose and cholesterol]. The molecular electrostatic potential (MEP) is determined as described in the literature (Chigo Anota et al. 2013c).
Results and discussion
Studies are reported of the following systems: graphene–monomer of chitosan, boron-doped graphene–monomers of chitosan, and boron-doped graphene–monomer of chitosan–glucose and boron-doped graphene–monomer of chitosan–cholesterol interactions (Fig. 1). For the presentation of results, we first consider the interaction of pristine graphene with the monomer of chitosan (G + MCh) in configurations: 1 the molecule is oriented perpendicular to the nanosheet, the interaction is through the oxygen of the piranosic group (C5O), 3 where the orientations are defined by the OH functional group, 1 perpendicular through the amine group (NH2) and 2 where the central hexagon of the graphene is parallel to the monomer. The following step considers the graphene functionalization with a boron atom to form an epoxy group with the (G + B) mesh; this is done to improve the G functionalization with chitosan. Finally, the adsorption of glucose (C8H12O6) and cholesterol (C27H46O) is explored on the G + B + MCh system to test for the sensing capability.
Graphene–chitosan interaction
It is well known that graphene displays extraordinary properties such as high thermal conductivity, mechanical flexibility and good biocompatibility. These make the system suitable for applications in biosensor devices of the electrochemical type (Pumera 2009, 2010; Allen et al. 2010; Long et al. 2012) and moreover allows the formation of devices free of metals (Kuila et al. 2011). Chitosan may be easily obtained from waste shrimp skeleton (Hernández Cocoletzi et al. 2009) and exhibits properties which include good biocompatibility and capability to form membranes suitable for applications (Kurita 2001; Masuko et al. 2005). Consequently in this work, we perform non-periodic molecular simulations to investigate the structural and electronic properties of graphene interacting with chitosan. The minimum energy criterion and the non-negative vibration frequency guarantee an energy minimum of the global potential energy surface. The process of finding the minimum energy is done for all structures. Results show that the lowest minimum energy corresponds to the structure where the monomer of chitosan is oriented perpendicular to the graphene nanosheet, with the interactions being through the amine group of the monomer and the central hexagon of the carbon mesh. The binding energy is −8.22 eV (chemisorption, Table 1) and a bond length of 3.9 Å (Fig. 1b). These results agree well with those obtained in the interactions of chitosan with the BN hexagonal nanosheet (Chigo Anota et al. 2013b).
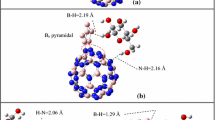
G nanosheet structural changes induced by the interaction with MCh show that C–C bond lengths take values in the interval of 1.39–1.42 Å, while at the same time the flat surface characteristic remains. In the monomer, the bond angle of the amine group H–N–H is 107.54° which is somewhat contracted as compared with the pristine case (102.15) (Chigo Anota et al. 2013d). In the C–C–O and C–C–C bond angles,contractions of 2.28° and 2.84°, respectively, are obtained again as compared with the pristine cases. Finally, the methyl group (CH2) exhibits an angle reduction of 0.51°.
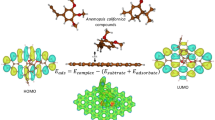
The G nanosheet polarity is 2.9 × 10−3 and the MCh polarity is 2.13 D; on the other hand, the G + MCh polarity is 3.49 D, which indicates that the monomer induces ionic characteristics in the G + MCh system, that is, produces the charge localization. The HOMO (with energy −4.73 eV) and LUMO (with energy −2.79 eV) isosurfaces with contributions from the hybridized carbon p z and s atomic orbitals are concentrated on the nanosheet (Fig. 2a, b). Calculations of the HOMO–LUMO energy difference (1.30 eV, Table 1) indicates that the system is a semiconductor, which is similar to the pristine graphene (Chigo Anota et al. 2013b). This means that the monomer makes no contribution to the HOMO–LUMO energy difference.
The analysis of the vibrations yields stretching oscillations at the wave number 4,092.46 cm−1 and bending at 4,181.10 cm−1 of the amine group of the monomer of chitosan. Stretching modes at wave numbers 3,655.83, 3,641.61 and 3,662.93 cm−1 of the piranosic hydrogen atoms are obtained. Stretching (at 3,699.15 cm−1), anti-stretching (at 2,048.63 cm−1) and scissor (at 2,265.29, 2,346.87, 2,338.16, and 2,472.9 cm−1) modes of the carbon and hydrogen atoms are found in the monomer, in agreement with the isolated G nanosheet oscillations where an anti-stretching mode at 264.63 cm−1 is also observed.
Boron-doped graphene–chitosan interaction
It has been reported recently the formation of graphene-chitosan films exhibit increased conductivity (attributed to the large surface-to-volume ratio and high conductivity of graphene) and electronic transference processes (Kang et al. 2009); therefore we explore conditions to improve the chitosan adsorption on the G nanosheet. To achieve this, nanosheet functionalization with boron atoms is proposed. This process allows the adsorption of chitosan near the impurity atom at one side of the central hexagon forming an epoxy-like configuration. The structure of minimum energy displays a curvature at the carbon mesh of 6.11° (Fig. 1c), and the bond distance between the G and boron, Cmesh–B, takes asymmetry values of 1.87 and 1.82 Å, respectively. A protrusion is formed at the zone and the hybridization changes from sp2 to sp3.
When the monomer of chitosan is adsorbed, a new B–N bond (with the nitrogen of the amine group of the monomer) is formed of length 1.57 Å. This value is similar to that reported in the adsorption of the amine group on the hBN nanosheet (Chigo Anota et al. 2013d) and in the monomer adsorption on BN nanotubes (Rodriguez Juárez et al. 2013) with different chirality through the boron atom. The adsorption energy is −8.60 eV (chemisorption, Table 1) which is increased as compared to the previous case (G + MCh); this may suggest the system to be used as a biomolecule sensor, which is due in part to the increment in the charge transfer as shown by the MEP surface.
When the G nanosheet is doped with boron, the molecular orbitals are localized at the vicinity of the Cmesh–B bond and at the new B–Namine bond (Fig. 2c, d). These orbital localizations indicate a charge transfer to the polysaccharide (Fig. 2e–h), which induces a better adsorption process. The charge distribution is indicated by the dipole moment direction (Fig. 3a, b) and also shown by the graphs of MEPs (Fig. 3c, d), where the yellow zone is for the negative charge. The large adsorption energy of the monomer on the graphene sheet is validated by the MEP surface which exhibits a charge distribution in the entire interaction zone (Fig. 3c), in a similar fashion as in the pristine graphene (Fig. 3c, inset depicted at the right upper corner).
The dipole moment of G + B + MCh + X system: X glucose (a) and X cholesterol (b). These values were calculated using the code Gaussian09 (Frisch et al. 2009). c The molecular electrostatic potential of G + MCh and G; d the corresponding electrostatic potential of G + B + MCh
This charge localization may be measured by Raman experiments, provided the vibrations are increased. The polarity suggests (as a measure of the charge transfer) that graphene functionalized with boron (Ling et al. 2010), deposited on chitosan, may be of practical use, provided the interaction with the biomolecules is favored.
It is important to realize that the molecular HOMO and LUMO frontier orbitals of the polysaccharides (insets at the upper corner of Fig. 2c–h) make no contribution to the G + B + MCh. The structural change induced by the bonding of the monomer with the boron atom of the nanosheet is evident at the H–N–H bond angle of the amine which is reduced by an amount of 1.18° with respect to the boron free structure. The monomer hexagon (piranosic group) is affected by changes in the bond angles: an increase of 2.25° at the C–C–C fragment and 0.63° at the C–C–O fragment. The methyl group experiences a small variation in the bond angle (of the order of 10−2).
Boron-doped graphene–monomer of chitosan–glucose and cholesterol
One possible application of the G + B + MCh system is as a biosensor (receptor); therefore in this section, we explore the glucose adsorption (Fig. 1g) which displays structural and chemical characteristics similar to the monomer of chitosan. Different geometries have been tested. In the first, the adsorbate interacts with the OH group, in the second, the interaction is through the methyl group; in the third and fourth, the glucose is parallel to the monomer of chitosan. The cholesterol (Fig. 1h) is in one case perpendicularly bound through the OH group of both systems, two configurations are with parallel orientations and in the last the cholesterol OH fragment is oriented toward the hexagon (piranosic group) of the monomer of chitosan. Minimum energy results yield the monomer of chitosan parallel to the structure with an adsorption energy of −1.62 eV which favors glucose detection (Fig. 1g; Table 1) and an adsorption energy of −2.58 eV for the cholesterol through the polysaccharide group (Fig. 1h; Table 1). The interaction distance is 2.41 Å in the G + B + MCh−Glucose (this is measured from one of the hydrogen atoms of the amine group in the monomer to the oxygen of the OH group in the glucose) and 2.47 Å (this distance measured from one of the hydrogen atoms in the amine group to one of the hydrogen atoms in the cholesterol) for the G + B + MCh + cholesterol and 2.76 Å for the cholesterol and G system. Structural changes are manifested mainly in the monomer of the chitosan, which exhibits a large inclination (Fig. 1g) with the monomer being attached to the G + B when cholesterol is adsorbed, and the amine group is contracted by an angle of 2.11°, the piranosic group at the C–C–O fragment is contracted by 0.49° and the C–C–C is contracted by 1.95°. Moreover, the bond angle at the methyl group is reduced by 0.18°. In the glucose case (Fig. 1h), in the methyl group the bond angle is increased by 0.12°, the C–C–O bond angle is increased by 0.39°, but the C–C–C bond angle is contracted by 1.16° and finally the bond angle at the amine group is reduced by 1.16°.
The vibrational modes in the G + B + MCh + glucose structure stretch at 37.05 cm−1 (this is from the polysaccharide toward the amine group), at 345.52 cm−1 for the vibration of the H–N fragment in the amine group toward one of the OH groups in the glucose, at 457.37 and 519.45 cm−1 in the bending vibration of the outer carbons. There are anti-stretching vibrational modes at 1,676.86 cm−1 in the vibrations between hydrogen atoms of the amine group, stretching modes at 1,924.94 and 3,722.79 cm−1 of the two hydrogen atoms in the amine group, and stretching modes at 2,372.34 cm−1 of the hydrogen atom bonded to the carbon in the C–C–C fragment of the piranosic group. Scissor vibration modes are at 2,561.88 cm−1 of the hydrogen atoms of the amine group in the monomer, and anti-stretching modes from 3,779.66 to 3,794.47 cm−1 of the outer hydrogen atoms. There are bending vibrations at 3,795.08, 3,801.09 and 3,813.39 cm−1 of the hydrogen atoms in the amine group and scissor vibration modes at 3,929.96 cm−1 of the methyl group in the monomer chitosan.
In the G + B + MCh + cholesterol structure, at 4,355.90 cm−1 the interaction of the cholesterol OH functional group with the carbon mesh occurs (stretching along the z-direction), at 50.67 cm−1 (stretching) the vibration of the chitosan monomer toward cholesterol is obtained, at 69.37 cm−1 the motion is of cholesterol toward MCh. At 4,114.13 cm−1, there is a stretching mode and at 4,114.13 cm−1 there is a bending mode of the amine group vibration which interacts with the polysaccharide. At 3,679.13 and 3,662.51 cm−1, there are stretching vibration modes of the amine group hydrogen atoms in the MCh toward the G nanosheet. This interaction is favored by the inclination of the monomer when cholesterol is adsorbed. Finally, the carbon stretching and anti-stretching mesh vibration modes are on the nanosheet plane.
Conclusions
We have presented studies of the interaction between the monomer of chitosan (MCh) and carbon nanosheets (graphene), the G + MCh system. The system has been modified by the functionalization of the carbon mesh with boron atoms (G + B) to form an epoxy-type group which bonds with the nitrogen of the amine group (this forms a new bond) to improve conditions in the detection of polysaccharides such as glucose and cholesterol. The interaction induces structural changes mainly in the monomer. The functionalized graphene nanosheet with chitosan (G + B + MCh) may be a better biosensor than the G + MCh system. This is corroborated by the larger value of the adsorption energy and by the vibrational motions of the amine group which bonds to the boron atom. This is further supported by the dipole moment direction which indicates charge transfer as both polysaccharides are bioadsorbed.
References
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110:132
Araujo V, Peñaranda M, Castellano O, Soscun H (2012) Propiedades estructurales, energéticas y electrónicas del complejo molecular formado por la interacción entre benceno y grafeno extendido: Investigación basada en la teoría del funcional de la densidad DFT. Ciencia 20(special issue):128–136
Boese AD, Handy NC (2001) A new parametrization of exchange-correlation generalized gradient approximation functionals. J Chem Phys 114:5497
Chigo Anota E, Ramírez Gutierrez RE, Escobedo Morales A, Hernández Cocoletzi G (2012) Influence of point defects on the electronic properties of boron nitride nanosheets. J Mol Model 18(5):2175–2184
Chigo Anota E, Escobedo Morales A, Salazar Vilanueva M, Vazquez Cuchillo O, Rubio Rosas E (2013b) On the influence of point defects on the structural and electronic properties of graphene-like sheets: a molecular simulation study. J Mol Model 19(2):839–846
Chigo Anota E, Ramírez Gutiérrez RE, Pérez Sánchez FL, Sánchez Ramírez JF (2013c) Structural characteristics and chemical reactivity of doped graphene nanosheets. Graphene 1(1):31–36
Chigo Anota E, Rodríguez Juárez A, Miguel Castro, Hernández Cocoletzi H (2013d) A density functional theory analysis for the adsorption of the amine group on graphene and boron nitride nanosheets. J Mol Model 19:321–328
Chigo Anota E, Hernández Rodríguez LD, Hernández Cocoletzi G (2013a) Influence of point defects on the adsorption of chitosan on graphene-like BN nanosheets. Graphene. doi:10.1166/graph.2013.1014
Delley B (1990) An all‐electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, Evmenenko G, Nguyen ST, Ruoff RS (2007) Preparation and characterization of graphene oxide paper. Nature 448:457–460
Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, Ferrari AC, Boukhvalov DW, Katsnelson MI, Geim AK, Novoselov KS (2009) Control of graphene’s properties by reversible hydrogenation: evidence for graphene. Science 323:610–614
Ezawa M (2013) Quantum percolation transition from graphene to graphane: graph theoretical approach. Nanomat Nanotechnol 3:1–6
Foresman JB, Frisch Æ. (1996) Exploring chemistry with electronic structure methods. 2nd Edn. Gaussian Inc., USA, p 70
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al. (2009) Gaussian09, Revision C.01-SMP. Gaussian Inc., Pittsburgh
Hernández Cocoletzi H, Águila Almanza E, Flores Agustín O, Viveros Nava EL, Ramos Cassellis E, Lee YC (2005) Obtención y caracterización de quitosano a partir de exoesqueletos de camarón. Sup y Vac 21(3):57–60
Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y (2009) Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Biolectron 25:901–905
Kuila T, Bose S, Khanra P, Mishra AK, Hoon Kim N, Le Hee J (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648
Kurita K (2001) Controlled functionalization of the polysaccharide chitin. Prog Plym Sci 26:1921–1971
Li X, Wang H, Robinson JT, Sanchez H, Diankov G, Dai H (2009) Simultaneous nitrogen doping and reduction of graphene oxide. J Am Chem Soc 131:15939–15944
Li B, Zhou L, Wu D, Peng H, Yan K, Zhou Y, Liu Z (2011) Photochemical chlorination of graphene. ACS Nano 5:5957–5961
Ling X, Xie L, Fang Y, Xu H, Zhang H, Kong J, Dresselhaus MS, Zhang J, Liu Z (2010) Can graphene be used as a substrate for raman enhancement? Nano Lett 10:553–561
Long J, Xie X, Xu J, Gu Q, Chen L, Wang X (2012) Nitrogen-doped graphene nanosheets as metal-free catalysts for aerobic selective oxidation of benzylic alcohols. ACS Catal 2:622–631
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura I, Lee YC (2005) Thiolation of chitosan attachment of proteins via thioether formation. Biomacromolecules 6:880–884
McAllister MJ, Li JL, Adamson DH, Schniepp HC, Abdala AA, Liu J, Herrera Alonso M, Milius DL, Car R, Prud’homme RK, Aksay IA (2007) Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem Mater 19:4396–4404
Nair RR, Ren WC, Jalil R, Diaz I, Kravets VG, Britnell L, Blake P, Schedin F, Mayorov AS, Yuan S, Katsnelson MI, Cheng HM, Strupinski W, Bulsheva LG, Okotrub AV, Grigoreva IV, Grigorenko AN, Novoselov KS, Geim AK (2010) Fluorographene: a two-dimensional counterpart of teflon. Small 6(24):2877–2884
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Pumera M (2009) Electrochemistry of graphene: new horizons for sensing and energy storage. Chem Rec 9:211–223
Pumera M (2010) Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev 39:4146–4157
Rodriguez Juárez A, Chigo Anota E, Hernández Cocoletzi H, Flores Riveros A (2013) Adsorption of chitosan on BN nanotubes: a DFT investigation. Appl Surf Sci 268:259–264
Schniepp HC, Li JL, Mcallister MJ, Sai H, Herrera Alonso M, Adamson DH, Prud’homme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110:8535–8539
Sofo JO, Chaudhari AS, Barber GD (2007) Graphane: a two-dimensional hydrocarbon. Phys Rev B 75:153401–153404
Valentini L, Cardinali M, Kenny JM, Prato M, Monticelli O (2012) A photoresponsive hybrid nanomaterial based on graphene and polyhedral oligomeric silsesquioxanes. Eur J Inorg Chem 2012:5282–5287
Wang WL, Kaxiras E (2010) Graphene hydrate: theoretical prediction of a new insulating form of graphene. New J Phys 12:1250121–1250127
Xue Y, Liu Y, Lu F, Qu J, Chen H, Dai L (2012) Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J Phys Chem Lett 3:1607–1612
Yaya A, Ewels CP, Suarez-Martinez I, Wagner PH, Lefrant S, Okotrub A, Bulusheva L, Briddon PR (2011) Bromination of graphene and graphite. Phys Rev B 83:0454111–0454115
Zhou J, Wang Q, Sun Q, Chen XS, Kawazoe Y, Jena P (2009) Ferromagnetism in semihydrogenated graphene sheet. Nano Lett 9(11):3867–3870
Acknowledgments
This work was partially supported by projects: VIEP-BUAP (CHAE-ING13-G), Cuerpo Académico Ingeniería en Materiales (BUAP-CA-177), Cuerpo Académico Física Computacional de la Materia Condensada (BUAP-CA-191) and VIEP-BUAP-EXC11-G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Chigo Anota, E., Torres Soto, A. & Cocoletzi, G.H. Studies of graphene–chitosan interactions and analysis of the bioadsorption of glucose and cholesterol. Appl Nanosci 4, 911–918 (2014). https://doi.org/10.1007/s13204-013-0283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0283-0