Abstract
Main objective of present work is to study the efficiency of mixed fuel towards solution combustion synthesis of alumina powder, which otherwise prepared by single fuel and study of properties of final product with mixed fuel approach. Two different fuels, glycine and urea, along with aluminium nitrates have been used to prepare nanophase alumina powder. Different fuel to oxidizer ratios and different percentage combination of two fuels were used to prepare six samples. In all samples, nanoscale particle size obtained. Parameter which continuously changes the results of various characterisations is percentage combination of two fuels. In case where percentage of urea is higher than glycine reaction takes place with high exothermicity and hence crystallinity in product phase, whereas glycine promotes amorphous character. With mixed fuel approach, crystallinity can be enhanced easily, by calcinations of powder product at low temperature, because due to mixed urea and glycine, there is already some fraction of crystallinity observed. Overall mixed fuel approach has ability to produce nanophase alumina powder with wide range of particles size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent times, the general procedure utilised by scientists to understand the science of materials consist of studying large and complex structures and then to investigate the basic building blocks of these microstructures that are smaller and simpler. This approach is often termed “Top-down” science. However, with the advent of scanning probe microscope, which permits keen observation of individual particles (atoms and molecules), it has become possible to rectify and move atoms and molecules to form simple atomic level constituents. This ability to arrange atoms provides opportunities to develop unique properties that are not otherwise possible. We term this the “bottom-up” approach, and the study of the properties of these materials is termed “nanotechnology” (Toniolo et al. 2005; Mimani and Patil 2009). Because of rare properties, nanometal oxides are useful as catalysts, sensors, coating materials, grinding media, abrasion resistant tiles, cutting tools, bioceramics (hip-joints), body armour, laboratory ware and wear parts for the textile and paper industries and many more (Bahadur et al. 2006; Patil et al. 2002).
Different methods are available for the synthesis of nanomaterials like sol–gel method, laser abrasion, chemical vapour deposition, co-precipitation, solution combustion synthesis. Solution combustion synthesis technique is an easy and time-saving production process (Lenka et al. 2007; Hwang and Wu Tsung-Yung 2004; Matteo and Sara 2007). Combustion synthesis process is based upon the thermochemical concept used in the field of propellants and explosives, its extrapolation to the combustion synthesis of nanooxides. Chemically, mechanism of combustion process is quite complex. But in spite of complexity in the process of combustion synthesis presents some advantages over other methods: reagents are simple compounds, formation of high purity product, stabilisation of metastable products, special equipment is not required and agglomeration of final powder product remains limited (Prakash et al. 2002; Merzhanov 1995). Combustion synthesis is a two-step process: formation of precursor and then auto-ignition. This auto-ignition is often termed as self-propagating high temperature synthesis. The processing parameters that effect the properties of final product are fuel to oxidizer ratio, type of fuel, amount of fuel percentage, ignition temperature, water content in precursor, ph of the solution. A good fuel should possess the properties like non-violent reactions and without evolution of toxic gases (Merzhanov 1996; Pathak et al. 2002). A number of fuels like urea, glycine, tetra formal tris azine, malonic acid dihydrazide, oxalyl dihydrazide are being used to prepare technologically important metal oxides. These fuels accomplish two purposes: during combustion forms CO2, H2O and liberate heat in exothermic process and forms complexes with metal ions facilitating homogeneous mixing of the cations in solution (Biamino et al. 2006; McKittrick et al. 1999). The exothermicity (Tad) of the redox reaction ranges from 1,000 to 1,800 K. Depending upon the fuel used, the nature of combustion differs from flaming to non-flaming (smouldering). In present experiment after studying the synthesis with urea and glycine, a new approach, mixed fuel approach (urea mixed with glycine), is used and effect of fuel to oxidizer ratio along with mixing proportion of two fuels is analysed (Sharma et al. 2012).
Experimental
Materials and methods
Aluminium nitrate non-hydrate (GR)
Aluminium nitrate is a strong oxidising agent. It is used in tanning leather, antiperspirants, corrosion inhibitors, extraction of uranium, petroleum refining and as a nitrating agent. The non-ahydrate and other hydrated aluminium nitrates have many applications. These salts are used to produce alumina for preparation of insulating papers, in cathode ray tube heating elements, and on transformer core laminates. The hydrated salts are also used for the extraction of actinide elements.
-
Symbol: Al(NO3)3·9H2O, molecular: weight 375 g/mol,
-
solubility in distilled water: 637 g/l at 25 °C, oxidising–reducing valency: −15
Urea
Urea or carbamide is an organic compound with the chemical formula CO(NH2)2. The molecule has two NH2 groups joined by a carbonyl (C=O) functional group. It is a colourless, odourless solid, although the ammonia that it gives off in the presence of water, including water vapour in the air, has a strong odour. Used as a fuel in synthesis of various oxides.
-
Symbol: CO(NH2)2, molecular weight: 60 g/mol,
-
solubility in distilled water: 1,080 g/l at 25 °C, oxidising–reducing valency: +6
Glycine
Glycine (abbreviated as Gly or G) is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its side-chain, glycine is the smallest of the 20 amino acids commonly found in proteins. Glycine is an intermediate in the synthesis of a variety of chemical products.
-
Symbol: C2H5NO2, molecular weight: 75 g/mol,
-
solubility in distilled water: 250 g/l at 25 °C, oxidising–reducing valency: +9
Stoichiometric oxidizer to fuel molar ratio
An oxidizer is a type of chemical which a fuel requires to burn. Here, in solution combustion process, we first should have the knowledge of amount of fuel required to complete the combustion process, which is termed as stoichiometric ratio. This ratio can be determined by calculating oxidising–reducing valency, which is the calculation of number of atoms removed and accepted by oxidizer and fuel during redox reaction.
-
(a)
Al(NO3)3 (oxidizer) and CO(NH2)2 (fuel):
$$ \begin{aligned} {\text{oxidizer}}/{\text{fuel}}\;{\text{ratio}} & = \sum {\text{all oxidizing}}\;\& \;{\text{reducing elements in oxidizer }} \\ & \quad \div \left( { - 1} \right) \, \sum {\text{ oxidizing}}\;\& \;{\text{reducing elements in fuel}} \\ & = \, 15/6 = 2.5 \\ \end{aligned} $$Hence, the stoichiometric aluminium nitrate/urea molar ratio is 1:2.5, required for the complete combustion of precursor.
-
(b)
Al(NO3)3 (oxidizer) and C2H5NO2 (fuel):
$$ \begin{aligned} {\text{oxidizer}}/{\text{fuel ratio}} & = \sum {\text{all oxidizing }}\& {\text{ reducing elements in oxidizer}}\\ & \quad \div \left( { - 1} \right) \, \sum {\text{ oxidizing }}\& {\text{ reducing elements in fuel}} \\ &= \, 15/9 = 1.66 \\ \end{aligned} $$Hence, the stoichiometric aluminium nitrate/glycine molar ratio is 1:1.66, required for the complete combustion of all material.
Chemical equations
-
(a)
Al(NO3)3 and CO(NH2)2
$$ 2{\text{Al}}\left( {{\text{NO}}_{3} } \right)_{3}\;+\;5{\text{CO}}\left( {{\text{NH}}_{2} } \right)_{2} \to {\text{Al}}_{2} {\text{O}}_{3}\;+\;5{\text{CO}}_{2}\;+\;10{\text{H}}_{2} {\text{O }}\;+\;8{\text{N}}_{2} $$ -
(b)
Al(NO3)3 and C2H5NO2
$$ 3{\text{Al}}\left( {{\text{NO}}_{3} } \right)_{3}\;+\;5{\text{ C}}_{2} {\text{H}}_{5} {\text{NO}}_{2} \to 3/2{\text{ Al}}_{2} {\text{O}}_{3}\;+\;10{\text{CO}}_{2}\;+\;12.5{\text{ H}}_{2} {\text{O}}\;+\;7{\text{N}}_{2} $$ -
(c)
For glycine to urea ratio 0.5:0.5
$$ 6{\text{Al}}\left( {{\text{NO}}_{3} } \right)_{3}\;+\;7.5{\text{CO}}\left( {{\text{NH}}_{2} } \right)_{2}\;+\;5{\text{C}}_{2} {\text{H}}_{5} {\text{NO}}_{2} \to 3{\text{Al}}_{2} {\text{O}}_{3}\;+\;17.5{\text{CO}}_{2}\;+\;27.5{\text{H}}_{2} {\text{O }}\;+\;19{\text{N}}_{2} $$ -
(d)
For glycine to urea ratio 0.75:0.25
$$ 6{\text{Al}}\left( {{\text{NO}}_{3} } \right)_{3}\;+\;11.25{\text{CO}}\left( {{\text{NH}}_{2} } \right)_{2}\;+\;2.5{\text{C}}_{2} {\text{H}}_{5} {\text{NO}}_{2} \to 3{\text{Al}}_{2} {\text{O}}_{3}\;+\;16.25{\text{CO}}_{2}\;+\; 28.75{\text{H}}_{2} {\text{O }}\;+\;21.5{\text{N}}_{2} $$ -
(e)
Thermal dissociation of Al(NO3)3
$$ 2{\text{Al}}\left( {{\text{NO}}_{3} } \right)_{3} \to {\text{Al}}_{2} {\text{O}}_{3} + 6{\text{NO}}_{2} + 3/2{\text{O}}_{2} $$
Precursor preparation
Present work involves, variation in combination of two fuels and oxidizer to fuel ratio.
To prepare precursor, we have
Forty-five grams of Al(NO3)3 are mixed with 45 ml of distilled water, dissolve to prepare homogeneous solution using magnetic steerer. Similarly, 9 g of urea in 9 ml of distilled water and 4.5 g of glycine in 18 ml of distilled water.
-
Volume of Al(NO3)3 solution = 96 ml, volume of urea solution = 21 ml,
-
Volume of glycine solution = 22.5 ml
-
Al(NO3)3 solution is divided in six equal parts 16 + 16 + 16 + 16 + 16 + 16 ml in six beakers.
In stoichiometry ratio, 7.5 g of Al(NO3)3 requires 2.5 g of glycine for complete combustion, and in Stoichiometry ratio, 7.5 g of Al(NO3)3 requires 3 g of urea for complete combustion.
In present experiment, six samples (1E, 2E, 3E, 4E, 5E, 6E) with different combinations of fuel to oxidizer ratios and combinations of two fuels were prepared (detail given in Table 1).
-
1E—Fuel lean, 2E—Fuel lean, 3E—Fuel lean
-
4E—Fuel lean, 5E—Fuel rich, 6E—Fuel rich
Mixing and furnace heating
All the precursor sample 1E, 2E, 3E, 4E, 5E, 6E, mixed for 2–3 min, using magnetic steerer, one by one. The each sample is subjected to furnace heating in the temperature range from 350 to 450 °C, until solution become free from water to form final foam-like product.
Sample preparation
All the six foamy products formed after solution combustion process are grounded thoroughly to form a homogeneous powder, without agglomerated particles. Powder is used for XRD and SEM characterisation.
Results and discussion
Visual flame observations
From flame observation during combustion reaction inside the furnace, we observed that (see Table 2), in case of sample 1E, which is fuel lean, gives white foamy product with negligible flame, which indicates that there is negligible exothermic reaction that takes place during combustion. Formation of foamy white product is due to the temperature dissociation of nitrate, not because of main fuel reaction. Formation of white product indicates some degree of combustion of precursor, which is due to urea. Similar observation obtained for the sample 2E, when 10 % of glycine and 30 % of urea (from stoichiometric value) is used, with more rising of foam as compared to sample 1E. In case of sample 3E, high red flame for 12–15 s was observed, and high rising of foam in beaker indicates higher extent of exothermicity in combustion reaction. In the same way for sample 4E, high flame was observed (see Fig. 1), with foamy product coming outside the beaker, which indicates higher exothermicity and thus higher combustion reaction temperature. In case of 4E, percentage of urea is more than glycine. Similarly, when we observe sample 5E, high raising of foamy product outside the beaker takes place. In this case, black foamy product is obtained, which is due to residual carbon content from glycine, as the percentage of glycine is higher in 5E, than 3E, 4E. In case of 6E, higher rising flame and rising foam outside the beaker are similar to 5E, but grey product is formed, which indicates high degree of exothermicity and thus greater extent of combustion of precursor.
From all above observations, we note that in case where percentage of urea is greater than glycine, excellent combustion, without residual carbon contents takes place. Whereas in case, when percentage of glycine is comparable to urea, black product is formed, which indicates incomplete combustion or combustion with residual carbon content in product phase. This reveals the fact that increasing urea in mix fuel approach increases the extent of exothermicity in solution combustion.
XRD analysis
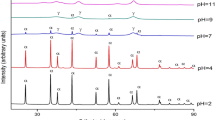
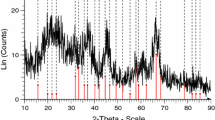
XRD patterns of the combustion product were recorded using a Siemens D500 with Cukα radiations. The XRD of the as-synthesized powder showing that in case of sample 1E (Fig. 2), containing 20 % of urea and 20 % of glycine complete amorphous product is formed. Similar result is obtained for the sample 2E (Fig. 3). Formation of amorphous phase is due to the lack of sufficient temperature required to promote alumina crystallisation. In case of sample 3E (Fig. 4), there is some fraction of crystallinity, which is due to increase in percentage of urea, which in turn increases the exothermicity and hence combustion temperature. In case of sample 4E (Fig. 5), the extent of crystallinity increases because of higher percentage of urea (60 %) in precursor. In case of sample 5E (Fig. 6), some crystallinity is observed, but amorphous character dominates, which is due to higher content of glycine, which results in amorphous product phase. In case of 6E (Fig. 7), crystallinity again increased due to higher percentage of urea than glycine and also due to fuel rich conditions. All above observations reveal the fact that in mixed fuel, urea promotes crystallinity and glycine promotes amorphous character of the product phase.
SEM analysis
From SEM analysis, we observed that there is presence of foamy agglomerated particles with a wide distribution (low magnification) and presence of larger particles in their structure (high magnification). Formation of these features is attributed to the amount of flame temperature during combustion process. In case of fuel lean sample 1E (Fig. 8), as also indicated by XRD analysis, that small amount of combustion reaction takes place with no flame, results in amorphous product and particle in the size range of 50–150 nm. This is due to evolution of gases during combustion reaction, which leads to amorphous structure. In case of sample 2E (Fig. 9), similar amorphous phase is obtained, with little increase in range of particle size. In case of sample 3E (Fig. 10), there is wide distribution of particle size, with formation of large particles up to 200 nm. Increase in particle size, attributed to efficiency of urea to produce high combustion temperature and thus to promote agglomeration of particles. As we proceed from sample 4E to 6E the percentage of urea in precursor increases, which results in increase of particle size and hence crystallinity. In case of 4E (Fig. 11), when urea is in higher percentage than glycine, the range of particle size increases up to 250 nm. Similarly in case of 5E and 6E (Figs. 12, 13), the range of particles size increases, because of higher percentage of urea compared to glycine and also due to fuel rich conditions. All above discussion indicates that urea promotes the high flame temperature and hence agglomeration of particles, gives large and wide range of particle sizes. Glycine results in small range of particle sizes. So in mixed fuel technique, there is formation of particles in size range of 50–300 nm.
Conclusion
Alumina powder (Al2O3) is prepared by using solution combustion technique, with mixed fuel (urea and glycine). From all the characterisations, we conclude that nanophase alumina powder is obtained in all fuel proportions, but in case of urea rich fuel, the extent of exothermicity is higher than in case when equal percentage of urea and glycine is used. Urea promotes crystalline character of the product, whereas glycine promotes amorphous character of the product. Also in mixed fuel approach, crystallinity can be enhanced easily by calcinations of powder product at low temperature because, due to mixed urea and glycine, there is already some fraction of crystallinity observssed. In mixed fuel, there is wide range of particle size in nanoscale, because urea promotes high flame and hence agglomeration of particles, whereas glycine promotes the formation of small range of particles size. Hence, in mixed fuel approach, the nanophase alumina powder was obtained with wide range of particles size. From overall discussion, we conclude that mixed fuel approach has an outstanding potential for producing crystalline alumina powder particularly in urea rich case, which highlights the potential of urea to promote crystallinity.
References
Bahadur D, Rajakumar S et al (2006) Influence of fuel ratios on auto combustion synthesis of barium ferrite nano particles. J Chem Sci 118(1):15–21
Biamino S et al (2006) Alumina–zirconia–yttria nanocomposites prepared by solution combustion synthesis. Ceram Int 32:509–513
Hwang C-C, Wu T-Y (2004) Combustion synthesis of nanocrystalline ZnO powders using zinc nitrate and glycine as reactants—influence of reactant composition. J Mater Sci 39:6111–6115
Lenka RK et al (2007) Combustion synthesis of gadolinia-doped ceria using glycine and urea fuels. J Alloys Compd. doi:10.1016/j.jallcom.2007.11.028
Matteo P, Sara B (2007) Mesoporous alumina obtained by combustion synthesis without template. J Porous Mater. doi:10.1007/s10934-007-9168-5
McKittrick J et al (1999) The influence of processing parameters on luminescent oxides produced by combustion synthesis. Displays 19:169–172
Merzhanov AG (1995) History and recent developments in SHS. Ceram Int 21:371–379
Merzhanov AG (1996) Combustion processes that synthesize materials. J Mater Process Technol 56:222–241
Mimani T, Patil KC (2009) Solution combustion synthesis of nanoscale oxides and their composites. Mater Phys Mech 4:134–137
Pathak LC et al (2002) Effect of pH on the combustion synthesis of nano-crystalline alumina powder. Mater Lett 57:380–385
Patil Kashinath C et al (2002) combustion synthesis: an update. Curr Opin Solid State Mater Sci 6:507–512
Prakash AS et al (2002) Hexamethylenetetramine: a new fuel for solution combustion synthesis of complex metal oxides. J Mater Synth Process 10(3):135
Sharma A, Modi OP, Gupta GK (2012) Effect of fuel to oxidizer ratio on synthesis of alumina powder using solution combustion technique—aluminium nitrate & glycine combination. Adv Appl Sci Res 3(4):2151–2158
Toniolo JC, Lima MD, Takimi AS, Bergmann CP (2005) Synthesis of alumina powders by the glycine-nitrate combustion process. Mater Sci Bull 40:561–571
Acknowledgments
First of all thanks to god, then to my parents who made me able to contribute my potential in research area. The author likes to convey sincere thanks to all staff in Material Science & Engineering section of AMPRI, Bhopal for their help and co-operation in completing this research work. A special thanks to my wife Mrs. Manu Sharma (Lecturer in Botany) for her motivation and inspiration to complete this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sharma, A., Rani, A., Singh, A. et al. Synthesis of alumina powder by the urea–glycine–nitrate combustion process: a mixed fuel approach to nanoscale metal oxides. Appl Nanosci 4, 315–323 (2014). https://doi.org/10.1007/s13204-013-0199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0199-8