Abstract
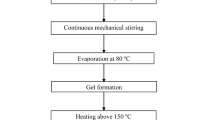
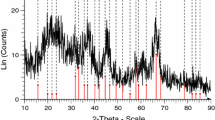
Nanocrystalline ceramic oxide particles with high purity can be synthesized efficiently by the solution combustion synthesis route, which is based on redox reactions between metal salts and reducing agents such as urea and glycine. In the present study, nanocrystalline alumina powders were synthesized using aluminium nitrate nonahydrate as the metal salt and urea as the fuel. The powders were characterized primarily for the phases present and crystallite size (from X-ray diffractometry) and morphology (by scanning electron microscopy). When stoichiometric amounts of the starting chemicals were taken, X-ray diffraction (XRD) analysis of the synthesized powder revealed it to be phase-pure α-Al2O3, with a crystallite size of 42 nm. Electron microscopy of the synthesized powder revealed a flaky morphology. Further, the pH value of the solution containing the stoichiometric amounts of the aluminium salt and the fuel was systematically varied by dissolving in liquid ammonia. It was observed (from XRD analysis) that an increase in the pH progressively stabilized the metastable γ-Al2O3 phase. An increase in the fuel (urea) content had no effect on the phase stability, but decreased the crystallite size of α-Al2O3. A crystallite size of 29 nm could be achieved with an excess fuel ratio of 1.5 over the stoichiometric value.




Similar content being viewed by others
References
Amato I, Nanotechnology––Shaping the World Atom by Atom, National Science and Technology Council, Washington DC (1999).
Gleiter H, Prog Mater Sci 33 (1989) 223.
Roco M, Am Inst Chem Eng J 50 (2004) 890.
Suryanarayana C, Adv Eng Mater 7 (2005) 983.
Hubner H, and Dorre E, Alumina: Processing, Properties and Applications, Springer, New York (1984).
Gitzen W H, Alumina as a Ceramic Material, Wiley, New York (1970).
Andersson J M, Controlling the Formation and Stability of Alumina Phases, Ph D Thesis, Linkoping University, Linkoping (2005).
Tok A I Y, and Zhao X L, J Mater Process Technol 178 (2006) 270.
Xiao Z L, Nano Lett 2 (2002) 153.
Zhang Y, J Nanomater 12 (2008) 122.
Hsing I, and Chuang C C, J Am Ceram Soc 90 (2007) 4070.
Laine R M, and Pan X Q, Nat Mater 5 (2006) 710.
Patil K C, and Mimami T, Curr Opin Solid State Mater Sci 6 (2002) 507.
Mimani T, Resonance 5 (2000) 50.
Mukasyan A S, and Peter D, Proc Combust Inst 31 (2007) 1789.
Das R N, Bandyopadhyay A, and Bose S, J Am Ceram Soc 84 (2001) 2421.
Pathak L C, Singh T B, Verma A K, and Ramachandrarao P, Mater Lett 57 (2002) 380.
Zhuravlev V D, Bamburov V G, and Grigorov I G, Ceram Int 39 (2013) 1379.
Baburao NS, and Umarji A M, Trans Indian Ceram Soc 70 (2011) 167.
Bhaduri S and Zhou E, Nanostruct Mater 7 (1999) 487.
Desilets S, Brousseau P, and Anderson J, Thermochim Acta 521 (2011) 59.
Karagedov G R, and Myz A L, J Eur Ceram Soc 32 (2012) 219.
Ghosh S K, Roy S K, Dattu S, and Basu D, Mater Sci Eng B 176 (2011) 14.
Silva M C, Costa A C F M, and Friestas N L, Mater Sci Forum 775 (2014) 687.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jolly, B.M., Ravi, S.K., Ipe, S. et al. Effect of Process Parameters on the Characteristics of Nanocrystalline Alumina Particles Synthesized by Solution Combustion Process. Trans Indian Inst Met 68 (Suppl 2), 147–151 (2015). https://doi.org/10.1007/s12666-015-0534-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0534-8