Abstract
The [O] [H] functionalization of carbon nanotube (CNT) was studied using oxygen–hydrogen (O2–H2) gas mixture direct current (DC) glow discharge plasma technique for cathode/CNT-anode separation of 0.10 ± 0.01 cm. O2 and H2 were fixed at flowrate of 10.0 ml/min in order to obtain gas mixture ratio of 1:1. During the (O2–H2) gas mixture DC glow discharge, current–voltage (I–V) characteristic of gaseous species studied for various settings of gas pressures 1, 2, 3 and 4 mbar. The voltage at gap between cathode/CNT and anode, a breakdown voltage, was identified as “functionalization voltage” (Vfunc). Vfunc was noticed responsible for functionalization of functional groups on sidewall of CNT. The Vfunc were recorded as 796, 707, 594, and 663 V for gas pressures of 1, 2, 3 and 4 mbar, respectively. The co-relation between Vfunc and gas pressure was identified as linear relationship. But a voltage obtained due to the CNT/Cathode fall shows exponential relationship with the gas pressures. The possibility of [O] [H] functionalization was proved using Fourier transmission infra-red (FTIR) spectroscopy. Hydroxyl (–OH), carboxyl (–COOH), and carbonyl (–C=O) functional groups were identical as identified in the FTIR spectra. The field emission scanning electron microscope images show significant changes in the morphology of CNT which proves that the DC gas discharge plasma is a possible technique for [O] [H] functionalization on the sidewall of CNT.
Similar content being viewed by others
Introduction
The discovery of carbon nanotube (CNT) in 1991 has unpaved (Iijima 1991) numerous approaches. Many investigations have been carried out to enhance diverse applications of CNT in chemical sensing (Pisco et al. 2008; Arben Merkoci et al. 2005), plastic transistors (Masashi Shiraishi et al. 2004), optoelectronic devices (Vanmaekelbergh et al. 2007), field emission (Jong Min Kim et al. 2000; Saito Yahachi Saito et al. 2002; de Jonge and van Druten 2003) and molecular electronics (Singh et al. 2006). Both single-walled carbon nanotube (SWCNT) and multi-walled carbon nanotube (MWCNT) are proven as considerable nanomaterials in these diverse applications. However the nature of CNT as agglomeration in bundle, the non-reactive and the presence of amorphous layer have inflicted their widespread use in many applications (Felten et al. 2005). Furthermore, insolubility in water, polymer resins and other solvents have showed difficulties in processing CNT into a thin film (Kakade and Pillai 2008). However, surface modification on CNT was found to be an efficient technique in introducing nanoparticle or functional groups; hydroxyl (–OH), carboxyl (–COOH) and ammonia (–NH3) on the sidewall of CNT.
The well-known surface functionalization on CNT involves process like altering the wetting and adhesion characteristics while improving dispersions of functional groups in the matrices of CNT. The attachment of functional groups on the sidewall of CNT without significantly changing the desirable properties of CNT provides a promising approach in its various fields of applications (Francesco Mercuri and Antonio Sgamellotti 2007). Conjunctionally, a number of research groups have demonstrated surface modification on CNT through both physical and chemical approaches, including polymer wrapping (O’Connell et al. 2001; Inna Gurevitch and Simcha Srebnik 2007), fluorination (Hamwi et al. 1997; Touharaa et al. 2002), mechano-chemical treatment (Konya et al. 2002; Chen et al. 2004) and plasma treatment (Bogaerts et al. 2002). Especially, the plasma treatment has exhibited advantage of non-polluting, which is not trifling for industrial fabrication. This technique also allows increase in quality and production quantity which are required for commercial use. In addition, the processing period of plasma treatment is shorter than any other techniques mentioned earlier. It also enables wide range dispersion of functional groups on the side wall of CNTs.
In this paper, we detailed the preparation of cathode/CNT-anode electrode system for DC gas discharge plasma treatment of H2 and O2 gas mixture. The processing parameter, gas pressures, was related with the Vfunc to show the –OH functionalization on the sidewall of CNT.
Experimental arrangement
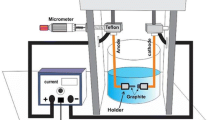
SWCNTs dissolved in an acetone solution were dispersed on silicon, Si(001) small chips cut into 1 by 1 ± 0.1 cm dimension. The Si(001) chips were cleaned in an ultrasonic bath of acetone solution to remove the presence of any oxide layers and impurities on the surface. The substrates further rinsed in deionised (DI) water to remove existing acetone solution. Three drops of CNT solution dispersed onto the cleaned silicon chip and further dried for 24 h in desiccators filled with drying agent, silica gel. This drying process could remove any moisture content from the CNT sample. A piece of non conductive tape was cut into a desired size and a small square hole 5 × 5 mm was placed at the centre of the tape. Then the tape was stuck onto the CNT sample and clamped to the brass electrode which acts as a cathode. The cathode/CNT was separated from an anode brass electrode using 1 mm thick glass slides. The prepared electrode system as shown in Fig. 1 was placed into a homemade stainless steel chamber that consists two sets of copper electrical feedthroughs. The chamber was then vacuumed to pressure about 10−5 mbar. The cathode/CNT and anode electrodes were connected to the high voltage supply via the copper electrical feedthroughs. O2 and H2 gases are flowed at gas mixture ratio of 1:1 into the vacuumed chamber. Potential difference from high voltage supply was slowly increased to maximum to obtain a current–voltage (I–V) characteristic of [O] [H] plasma between the cathode/CNT and anode for gas pressures of 1, 2, 3 and 4 mbar. The I–V characteristics were used to study and evaluate the [O] [H] functionalization on the sidewall of CNTs. The plasma-treated CNTs were compared with the non-plasma-treated CNT using FESEM (Quanta 200F) and FTIR spectroscopy.
Illustration of electrode system inside the home made stainless steel chamber. The electrode system consists of cathode brass electrode, anode brass electrode, two glass slides and Si (001) substrate contents of CNT shows the cathode/CNT-anode separation of 0.10 ± 0.01 cm. The electrical connection setup consists of 5 kV power supply, ammeter, and voltmeter
Results and discussions
FESEM
Figure 2 exhibits a comparison of FESEM images of non-plasma-treated CNT (Fig. 2a) and plasma-treated CNT (Fig. 2b, c, d) which reveals structural and morphological changes on the surface of CNTs. The morphology of non-plasma-treated CNT shows that almost all CNTs are not aligned in parallel to one another and found to be intersecting one another to form agglomeration in bundle. The CNTs are visible and randomly oriented and spread on the Si(001) exhibiting small grooves and small bumps of CNT bundles. But this morphology has been slightly altered after treated with DC gas discharge plasma of O2 and H2 at the gap of cathode/CNT and anode. Figure 2b shows morphology of CNT after the DC gas discharge plasma for gas pressure 1 mbar. The morphology of CNTs bulging outward forming larger grooves and larger protuberant are vertically aligned from the Si substrate. Figure 2b1 shows magnified version of Fig. 2b. A closer look at the image reveals formation of flashes that cover large surface of CNT and few CNTs in a range of 10–15 nm joint together at point T (refer Fig. 2b1) and formed a bundle of CNTs. Even two CNTs are aligned in parallel. Figure 2c, c1 (magnified image) shows morphology obtained after DC gas discharge plasma for gas pressure of 2 mbar. Larger protuberant is visible and more flashes cover the surface of CNTs compared to Fig. 2a1 (non-plasma treated), b1 (plasma treated at 1 mbar). Figure 2d, e shows DC gas discharge plasma treated at pressures of 3 and 4 mbar, respectively. The respective magnified image of Fig. 2d, e is shown as Fig. 2d1, e1 exhibits formation of flashes that cover larger surface area of CNT. Comparatively, Fig. 2d1, e1 shows flashes that cover larger surface area than the Fig. 2b1, c1.
FTIR
Figure 3 shows significant changes in the FTIR spectra of non-plasma-treated CNT and plasma-treated CNT at gas pressures of 1, 2, 3 and 4 mbar. The spectra of plasma-treated CNTs are compared to non-plasma-treated CNT spectrum to identify the possibility of chemical bonding in the matrices of CNT. Non-plasma-treated CNT only exhibits C–H vibration mode around 2,857 and 2,917 cm−1. The CNT samples treated by DC gas discharge plasma of O2 and H2 at gas pressures of 1, 2, 3 and 4 mbar show broaden peak around 3,400 cm−1 that ascribes the –OH stretching vibration (Men et al. 2008; Hsin et al. 2006). Presence of peak at wavenumber 2,850 and 2,917 cm−1 shows the presence of C–H stretching frequencies (Niu et al. 2007) which correspond to alkyl groups in both plasma-treated CNT and non-plasma-treated CNT. The intensity of the C–H stretching peak noticed to be increasing as the gas pressures increased from 1 to 3 mbar. But for gas pressure of 4 mbar the two peaks of C–H vibration further stretched and broadened to –OH broaden peak to form –COOH stretching vibration mode. Besides this, strong vibration mode at 1,728 cm−1 shows the evidence of –C=O chemical bonding in the matrices of CNT treated with DC gas discharge plasma for gas pressures of 4 mbar only.
I–V characteristics
Figure 4 shows the I–V characteristics of the CNT samples for various settings of gas pressures 1, 2, 3 and 4 mbar. The I–V characteristics are not smooth, indicating instabilities of emission current, presumably from cathode bombardment during discharge of O2 and H2 gases at the gap of cathode/CNT-anode. The breakdown voltages are identified as 796, 707 and 594 V for gas pressures of 1, 2, and 3 mbar show a descending trend as the pressures increased. But the breakdown voltage for gas pressure at 4.0 mbar shows higher voltage (663 V) than at the 3.0 mbar (594 V). I–V characteristics of 1, 2, 3 and 4 mbar show similar trend. Immediately after the breakdown voltage of the gas mixture, a sharp increase in voltage and current are seen at (815 V, 115 μA) and (667 V, 114 μA) for the gas pressures of 1.0 and 4.0 mbar. As the potential difference is increased, the gap voltage of cathode/CNT-anode shows sudden decrease to 660 to 580, 518 and 512 V for the respective gas pressures of 1, 2, 3 and 4 mbar. Further increase in potential difference of high voltage supply has exhibited proportional relation between the current and gap-voltage.
Discussion
The I–V characteristic of (O2–H2) gas mixture DC glow discharge at the cathode/CNT-anode separation is discussed based on the typical I–V characteristics reported for DC gas discharge plasma of Argon (Bogaerts et al. 2002). A very slight increase in current (almost constant current) is noticed in the gap between cathode/CNT-anode brass electrodes for the increased voltage of main supply. The saturation regime is almost unnoticeable due to a very low current <10−10 A. This explains that emissions of primary electrons from the CNTs (attached to cathode brass electrode) instead of cathode brass are due to the omnipresent cosmic radiation. It is suggested that active sides C+, C2+, and C3+ on the exterior sidewall of CNTs (C→Cn+ + e−; where n = 1, 2, and 3) are created. The combination of saturation and Townsend regime is a non-selfsustaining regime due to low electric field across at the gap of cathode/CNT-anode. At this regime, ionization of O2 and H2 could not be established. However, increasing the main voltage supply to the gap above 180 V has caused a transition from non-selfsustaining discharge to selfsustaining discharge. This could be due to the increased electric field at the gap of cathode/CNT-anode. At higher electric field, O2 and H2 are ionized by the impact of emitted primary electrons from CNTs (Bogaerts et al. 2002). The primary electron collides inelastically with O2 gas to form oxide ions which ascribed as O2 breakdown (O2 + 4e−→2O2−) whilst H2 breaks down into positive ions (H+) and electrons. These ion species (O2− and H+) chemically could attach to the active sites of sidewall of CNT to bond with functional groups through the phenomena called as “functionalization”. During the functionalization, the gap voltage of cathode/CNT-anode marked as gas breakdown voltage which is not selfsustainable and not visible showed a sudden drop to normal glow voltage. Therefore, the breakdown voltage can be referred as functionalization voltage (Vfunc).
The Vfunc and normal glow voltage are further analyzed (refer Fig. 5) as per detailed in Table 1. Figure 5 shows the corresponding relationship between the functionalization voltage and pressures. The Vfunc decreases linearly to the gas pressure ranged from 1.0 to 4.0 mbar. This explains that by setting the gas pressure to higher value, the presence of number of gas particles density per cm3 at the gap has increased and distance between the particles is decreased. This allows the particles to collide inelastically and more frequently with each other at the low gap voltage.
Based on the FESEM images, CNTs are aligned in parallel possibly due to electron transfer between the tip of CNT and the cathode brass electrode. Moreover, the hydrophobic surface of CNTs leads to repulsion among the tubes (Kakade and Pillai 2008) and holds the CNTs almost straight towards the cathode brass electrode. Formation of flashes increases as the gas pressure increases. The flashes indicate strong intertubular electrostatic interaction among the surface functional groups that hold SWCNTs to form multi-bundle SWCNTs. A significant change in diameter of nanotube is observed that confirms the formation of multi-bundle SWCNTs. Formation of flashes has joined the tips of the CNTs (Agrawal et al. 2006). This signifies an indication of using the DC glow discharge plasma to smoothen the top layer of CNTs.
Conclusions
[O] [H] functionalization of CNT using (O2–H2) gas mixture DC glow discharge plasma technique is obtained at cathode/CNT-anode separation of 0.10 ± 0.01 cm. The I–V characteristics of (O2–H2) gas mixture DC glow discharge showed breakdown voltages 796, 707, 594, and 663 V for gas pressures of 1, 2, 3 and 4 mbar. The breakdown voltage is identified as “functionalization voltage” that responsible for the functionalization of functional groups on the sidewalls of CNTs. FTIR has confirmed structural modification on the sidewalls of CNT which corresponds to increasing in the intensity of –OH stretching mode that broadens from 3,200 to 3,600 cm−1. Increased (O2–H2) gas mixture pressure up to 4 mbar has functionalized the sidewalls of CNTs with –COOH and –C=O functional groups. FESEM images proved the formation of bulging and grooves after the applied voltage between cathode/CNT and anode.
References
Agrawal S, Raghuveer MS, Kroger R, Ramanath G (2006) Electrical current-induced structural changes and chemical functionalization of carbon nanotubes. J Appl Phys 100:094314-1/5
Bogaerts A, Neyts E, Gijbels R, van der Mullen J (2002) Gas discharge plasmas and their applications. Spectrochimica Acta Part B 57:609–658
Chen Y, Conway MJ, Fitz Gerald JD, Williams JS, Chadderton LT (2004) The nucleation and growth of carbon nanotubes in a mechano-thermal process. Carbon 42:1543–1548
de Jonge N, van Druten NJ (2003) Field emission from individual multiwalled carbon nanotubes prepared in an electron microscope. Ultramicroscopy 95:85–91
Felten A, Bittencourt C, Pireaux JJ, Van Lier G, Charlier JC (2005) Radio-frequency plasma functionalization of carbon nanotubes surface O2, NH3, and CF4 treatments. J Appl Phys 98:074308-1/9
Gurevitch I, Srebnik S (2007) Monte Carlo simulation of polymer wrapping of nanotubes. Chem Phys Lett 444:96–100
Hamwi A, Alvergnat H, Bonnamy S, Beguin F (1997) Fluorination of carbon nanotubes. Carbon 35:723–728
Hsin YL, Lai JY, Hwang KC, Lo SC, Chen FR, Kai JJ (2006) Rapid surface functionalization of iron-filled multi-walled carbon nanotubes. Carbon 44:3328–3335
Iijima S (1991) Helical microtubes of graphitic carbon. Nature 354:56–58
Kakade BA, Pillai VK (2008) An efficient route towards the covalent functionalization of single walled carbon nanotubes. Appl Sci Surf 254:4936–4943
Kim JM, Choi WB, Lee NS, Jung JE (2000) Field emission from carbon nanotubes for displays. Diam Relat Mater 9:1184–1189
Konya Z, Vesselenyi I, Niesz K, Kukovecz A, Demortier A, Fonseca A, Delhalle J, Mekhalif Z, Nag JB, Koos AA, Osvath Z, Kocsonya A, Bir LP, Kiricsi I (2002) Large scale production of short functionalized carbon nanotubes. Chem Phys Lett 360:429–435
Men XH, Zhang ZZ, Song HJ, Wang K, Jiang W (2008) Functionalization of carbon nanotubes to improve the tribological properties of poly(furfuryl alcohol) composite coatings. Compos Sci Technol 68:1042–1049
Mercuri F, Sgamellotti A (2007) Theoretical investigation on the functionalization of carbon nanotube. Inorg Chim Acta 360:785–793
Merkoci A, Pumera M, Llopis X, Pérez B, del Valle M, Alegret S (2005) New materials for electrochemical sensing: carbon nanotube. Trends Anal Chem 24:826–838
Niu L, Luo Y, Li Z (2007) A highly selective chemical gas sensor based on functionalization of multi-walled carbon nanotubes with poly(ethylene glycol). Sens Actuators B 126:361–367
O’Connell MJ, Boul P, Ericson LM, Huffman C, Wang Y, Haroz E, Kuper C, Tour J, Ausman KD, Smalley RE (2001) Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem Phys Lett 342:265–271
Pisco M, Consales M, Cutolo A, Cusano A, Penza M, Aversa P (2008) Hollow fibers integrated with single walled carbon nanotubes: Bandgap modification and chemical sensing capability. Sens Actuators B 129:163–170
Saito Y, Hata K, Takakura A, Yotani J, Uemura S (2002) Field emission of carbon nanotubes and its application as electron sources of ultra-high luminance light-source devices. Phys B Condens Matter 323:30–37
Shiraishi M, Takenobu T, Iwai T, Iwasa Y, Kataura H, Ata M, Masashi S (2004) Single-walled carbon nanotube aggregates for solution-processed field effect transistors. Chem Phys Lett 394:110–113
Singh KV, Pandey RR, Wang X, Lake R, Ozkan CS, Wang K, Ozkan M (2006) Covalent functionalization of single walled carbon nanotubes with peptide nucleic acid: nanocomponents for molecular level electronics. Carbon 44:1730–1739
Touharaa H, Inahara J, Mizuno T, Yokoyama Y, Okanao S, Yanagiuch K, Mukopadhyay I, Kawasaki S, Okino F, Shirai H, Xud WH, Kyotani T, Tomita A (2002) Property control of new forms of carbon materials by fluorination. J Fluor Chem 114:181–188
Vanmaekelbergh D, Houtepen AJ, Kelly JJ (2007) Electrochemical gating: a method to tune and monitor the (opto) electronic properties of functional materials. Electrochim Acta 53:1140–1149
Acknowledgments
We gratefully acknowledge funding support from the Peruntukan Penyelidikan Pascasiswazah (PPP) of University Malaya, Grant No.: PS212/2009A.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Thandavan, T.M.K., Wong, C.S., Gani, S.M.A. et al. [O] [H] functionalization on carbon nanotube using (O2–H2) gas mixture DC glow discharge. Appl Nanosci 2, 47–53 (2012). https://doi.org/10.1007/s13204-011-0040-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0040-1