Abstract
Light alkane aromatization for aromatic compound production, used in petrochemical industries is an attractive area of research. The effect of second metal co-impregnation was investigated in stabilizing zinc on ZSM-5 in aromatization of propane. HZSM-5 was modified with zinc and iron metal by co wet-impregnation and characterized using XRF, XRD, BET, N2-adsorption, FTIR, FTIR-Pyridine, SEM, TEM, H2-TPR and XPS. The effect of different loadings of Iron on Zn/ZSM-5 was investigated on acidity, aromatic yield, product distribution and aromatization performance. Performance test was conducted in a fixed bed reactor at 540 °C, one atmosphere. GHSV of 1200 mL/g-h. Co-impregnation of Zn with Fe improved the catalytic activity and aromatic yield for 10 h time on stream as compared to parent HZSM-5 and Zn/ZSM-5 of very low aromatic yield and propane conversion. Impregnation of Zn as the dehydrogenating metal on HZSM-5 steadily increased aromatic yield from 5% on HZSM-5 to 25% and was steadily dropped to 20% after 10 h TOS. The co-impregnation of iron of 1–3 wt% loading as the second metal for zinc stability with 2 wt% Zn on ZSM-5 improved propane conversion and aromatic yield to 55% for the 10 h TOS. This further enhanced aromatic product distribution and minimized light gases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of light alkanes as industrial feedstock for petrochemicals and chemical plants has made its studies for aromatization a research interest both in industries and academia. Alkanes alongside other hydrocarbons are found in large deposits in natural gas reservoirs, gas condensate wells and as bye-products of petroleum refining operations. Conversion of propane into higher value chemicals via aromatization is of major importance [1].
ZSM-5 has unique structural pores that favor migration of aromatics due to their similarities in pore size [1,2,3], which enhances its high shape selectivity towards aromatics. Use of parent ZSM-5 as reported in literatures showed high coking and low selectivity towards aromatics as its limitations. Many works have been published towards mitigating this limitation via use of promoters, changing the silica alumina ratio to optimize the number and density of active sites, modifying the surface through hierarchical catalyst synthesis, shortening the path length via synthesis of nano-crystalline ZSM-5 etc [4,5,6,7,8].
Screening of several metals as dehydrogenating promoters have been studied on ZSM-5 support matrix to improve selectivity towards aromatics. Zinc, platinum and gallium metals have proven to be highly dehydrogenating though with some shortcomings to their use. Platinum metal is very expensive and prone to hydrogenolysis. The high cost of gallium metal alongside its hazardous nature to human health had cautioned its use as dehydrogenating metal. The high cost of gallium metal alongside its hazardous nature to human health had cautioned its use as dehydrogenating metal. Zinc metal is relatively cheap, readily available for procurement, highly dehydrogenating as bifunctional sites on ZSM-5 for light alkane aromatization but loses its stability at reaction temperature and long hour time on stream operations. In the case of metals on zeolites, it has also been reported that cations from transition metals can interact both with the Zinc atoms and the zeolitic structure, which helps to diminish or minimize the migration of metallic particles [9,10,11,12,13,14,15,16,17,18,19].
This present study investigated the co-impregnation effect of zinc metal as active site with different loading of iron on ZSM-5 for propane aromatization in fixed bed reactor.
Experimental section
Catalyst preparation
NH4-ZSM5 (Si/Al = 50) was sourced commercially from Sigma Aldrich and calcined to obtain HZSM-5 at 550 °C for 5 h. Aqueous solutions containing of Zn(NO3)2 as precursor for 2 wt% ZnO and Fe(NO3)2 as precursor for 1–3 wt% of Fe2O3 were co-impregnated by introducing them at the same time dropwise on solution of H-ZSM5 catalysts with stirring for effective mixing, and heated to dryness at 70 °C. The catalysts were further dried at 80 °C for 16 h and then calcined by temperature programmed furnace at 550 °C (4 °C per minute ramping) for 5 h. The reactions for iron(II) oxide and zinc oxide conversion from their precursor hexahydrates are given in Eqs. (1) and (2).
Characterization
FTIR spectra measurement was performed using Shimadzu FTIR-8400s spectrophotometer (64 scans, 4 cm−1) to obtain the characteristic spectra functional group. Pyridine-FTIR was used to identify and quantify the presence of Brønsted and Lewis acidity using Shimadzu FTIR-8400s spectrophotometer. Catalyst crystallinity was determined using the XRD patterns obtained using MiniFlex II X-ray diffractometer by Rigaku (Copper Kα rays, 40 kilovolts and 40 mill ampere, Kalpha1 = 1.540598, Kalpha2 = 1.544426 and Kbeta = 1.392218, 2q from 3° to 70° at scanning speed of 12° per minutes). N2-adsorption was conducted using Micrometrics ASAP-2020 machine at 77 K. Catalyst was degassed in a vacuum for 10 h at 300 °C. BET model was used to calculate the specific area of catalyst surface from the nitrogen isotherm. Analysis from t-plot were used to measure the micropore volumes and areas [11, 12]. Morphology of the catalyst surface were obtained using SEM Machine Hitachi S-4800 15 kilovolts. Transmission electron microscopy(TEM) images of the catalysts were obtained on a FEI Tecnai G2 Spirit STEM microscope (740 kilovolts using a carbon coated holey film on catalysts). Hydrogen-TPR plots of catalysts were conducted using Micrometrics equipment, at β = 10 °C/min; w = 75 mg; F = 50 ml/min and ∆T = 100–800 °C. XPS spectra were obtained with Axis Ultra Dld X-ray photoelectron spectrometer monochromatized micro-focused at AlKalpha-radiation, 15 kilovolts, 30 mA (450 W).
Catalytic activity evaluation
Activity tests of the prepared catalysts were carried out in a stainless-steel fixed bed continuous flow reactor with internal diameter of 9 mm. 0.5 g of the catalyst was mixed with 0.5 g of silica glass beads. The catalysts were degassed under inert nitrogen flow environment as the temperature rose through the reactor to 540 °C for 2 h before the reaction. Propane was introduced to flow through the reactor. The reaction was carried out at 540 °C and gas hourly space velocity (GHSV) of 1200 ml/g-h, pure propane and nitrogen of ratio 1:2 under atmospheric pressure. The products flowed from the lagged reactor product line to keep it in gaseous phase before entering the GC. The products from the reactor were analyzed in-situ using an online Buck Scientific gas chromatographer GC 910 attached to the reactor. The GC had thermal conductivity detector (TCD) with a Mol sieve 13X packed column for hydrogen, nitrogen and hydrogen detection and a flame ionization detector (FID) connected to Restek MTX1 and HAYESEP D oven packed column for hydrocarbon detection and analysis. Aromatic compounds and other products yield and selectivity were calculated based on the relative ratio of each product with respect to total moles of carbon containing products from the reactor.
Results and discussion
This work aimed at studying the effect of the iron second metal co-impregnation with zinc on ZSM-5. So comparison was made on parent ZSM-5, Zn/ZSM-5 and Zn–Fe/ZSM-5 catalyst of different iron loading.
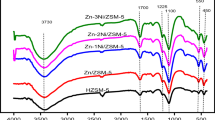
Figure 1 shows the FTIR spectra of HZSM-5 and the corresponding zinc and iron metal loaded catalysts. Basic HZSM-5 peaks were featured on the modified catalyst as they were on HZSM-5. All the characteristic peaks corresponding to parent HZSM-5 were present on the metal modified catalysts irrespective of loading. Peak intensities at 450 and 550 cm−1 depicted T–O bend and double-5 ring of crystalline of ZSM-5 while 1100 and 1225 cm−1 correspond to internal and external asymmetric stretch respectively. The peaks at 3400 and 1700 cm−1 were double bond stretching and bending vibration of hydroxyl (OH−) group of physically absorbed water on the surface of HZSM-5 zeolite [13,14,15, 20].
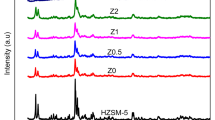
The X-ray diffractogram of the parent HZSM-5 with the metal modified catalysts were presented in Fig. 2. The loading of Zn and Fe had no significant effect on the HZSM-5 structure as loading increased except for the suppressed peak as a result of metal impregnated on the zeolite surface. This indicate that ZSM-5 crystallinity was maintained and structure unaffected by metals. This further confirmed that all the catalysts had the five typical distinguishing diffraction peaks of ZSM-5 structure suggest that metal impregnation process did not visibly change the framework structure of parent HZSM-5. There were no characteristic peaks to denote the present of the impregnated metals because of low concentration of metal zeolite surface and pore [16,17,18]. The characteristic peaks of ZSM-5 were found though suppressed at 2θ = 7.89°, 8.73°, 14.82°, 23.04°, 23.86°, and 24.26° at increased metal loading [19,20,21,22,23,24].
FTIR-Pyridine indicating the strength of the catalyst acidity with increase metal loadings was as shown in Fig. 3. It was observed that there were three major adsorption peaks of pyridine at approximately 1450 cm−1, 1490 cm−1 and 1540 cm−1, which were assigned to the characteristic bands of LAS, the cocontribution of LAS and BAS, and BAS, respectively. It was generally observed that the Bronsted acidity decreased as metal loading increased on parent ZSM-5. This was due to replacement of Bronsted sites by the added metals on the surface of the catalyst having a significant effect on activity test for propane aromatization [25, 26].
Nitrogen adsorption and desorption isotherm plots were shown in Fig. 4 for all the prepared catalysts. All the plots for the catalysts exhibited a Type I isotherm with high level microporosity as revealed in the BET specific surface area. The summary of the surface area and micropore analysis is presented in Table 1. Addition of Zn to the parent HZSM-5 decreased the total surface area. The specific total surface area, micropore and mesopore surface area of the catalyst were lower than HZSM-5 zeolite. These changes were due to the deposition of large Zn particles on the external surface, which blocked both the meso- and micropore. Zn–Fe/ZSM-5, the micro and meso-surface and pore volume increased. This suggests that some Fe particles attached to the external acid sites on the ZSM-5 structure while some on the surface [8, 27,28,29].
The SEM and TEM images study of micro-structured zeolitic catalyst were presented in Fig. 5. The morphology of parent ZSM-5 a did not vividly change after the addition of metallic oxides as the Zn and Fe were co-impregnated on HZSM-5 as seen from a-c which are SEM images of HZSM-5, Zn/ZSM-5 and Zn–Fe/ZSM-5 respectively. This implies that the metal addition had little influence on the surface morphology of HZSM-5 [30, 31]. The voids were filled on the TEM images as Fe aided even dispersion of Zinc on ZSM-5 matrix in Fig. 5d–f which also are TEM images of HZSM-5, Zn/ZSM-5 and Zn–Fe/ZSM-5 respectively.
The TPR profile in Fig. 6 obtained for HZSM-5 and Zn/ZSM-5 showed no peaks that could be attributed to H2-consumption. Fe/HZSM-5 contained double peaks [32], with maximum reducing temperature of 350 °C and 580 °C, which indicated that the reduction takes place in two stages. Zn–Fe/ZSM-5 had slight reduction around 390 and 560 °C [24, 33, 34]. It was suggested that a strong interaction between the metals led to shift in peak either to left or right from the normal single metal hydrogen-TPR thus enhancing formation of stable metal–metal alloy of Zn–Fe on ZSM-5. This also facilitated the reduction of hardly reducible metals like zinc. This occurrence could relate to the electronic interaction between Zn and Fe. In broad view, the strongest metal–metal interface occurs in catalyst systems between a metal with its valence band almost or completely filled and another metal with its valence band almost empty. Thus, the rearrangement of charge moves the electrons from Zn into the interface region between Zn and Fe [35].
The XPS analysis was employed often to obtain the surface composition details and oxidation states of metals of industrial catalysts [36]. Figure 7 showed Zn2+ and Fe2+ oxidations states. HZSM-5 and Zn/HZSM-5 zeolite were characterized by XPS. Figure 7c, the Fe 2p3/2 and Fe 2p1/2 XPS spectrum at 717.2 and 730.1 eV respectively could be attributed to the Fe2O3 phase, which suggested that a portion of clusters loaded in the surface of Zn–Fe/ZSM-5 were Fe2O3 species. The XPS spectra of Zn 2p3/2 and Zn 2p3/2 were observed at binding energy 1029 and 1051 eV respectively in Fig. 7b for Zn–Fe/ZSM-5 and 1025 and 1048 for Zn/ZSM-5 in Fig. 7a. The lower binding energy of Zn 2p3/2 showed the ZnO clusters dispersed in the surface of zeolite and the higher Zn 2p1/2 binding energy was attributed to the ZnOH+ species [25, 37]. Fe loading enhanced the dispersion of ZnO and increased the concentration of ZnOH+ which was also confirmed by XPS spectra in Fig. 7. It’s been researched that the ZnOH+ improved alkane aromatization and suppressed the formation of light olefins [38, 39]. The shift in the binding energy of Zn (1021.7 and 1045 eV) and Fe (710 and 725 eV) lower and higher energy level respectively from their basic oxides when compared to their binding energies in bimetallic form on ZSM-5 were attributed to inter-metallic bonding and electron balancing on zeolite surface and dispersion of zinc on zeolite matrix as shown from the XRD, SEM and TEM images.
Catalytic performance
Catalytic conversion of propane was performed at a reaction temperature of 540 °C and 1 atm. Conversion of propane to aromatics involves a series of reaction steps which include: dehydrogenation of propane to propene, oligomerization of formed alkenes from dehydrogenation, subsequent cracking (alkenes further conversion), new oligomers formation through alkenes alkylation followed by a hydrogen transfer and dehydrogenation, and cyclization and aromatization [6, 23, 29, 34, 40]. Dehydrogenation of propane is one of most important process of propane aromatization as it begins the reaction steps upon which subsequent reaction depends. It had been established that acidity of dehydrogenating metal-modified ZSM-5 is a vital factor affecting catalytic conversion of alkanes in heterogeneous catalysis reaction and corresponding selectivity towards aromatics [40, 41]. Figure 8 showed the effect of the Iron and zinc loading on parent ZSM-5. Propane conversion on HZSM-5 was high leading to formation of lighter gases and cyclohexane through cracking. Zinc had no much influence on conversion when compared with activity on HZSM-5. The presence of zinc helped in the recombination of surface hydrogen from hydride transfer steps. This occurred (working synergistically with the Bronsted sites of the HZSM-5) in propane dehydrogenation, oligomers and cyclic alkenes and dienes dehydrogenation, thus improving aromatic yield. It had been established in previous researches that aromatization on Zn/ZSM-5 catalysts occurs mainly via a bifunctional mechanism [6, 23, 25, 27]. The species of zinc present formed the Lewis sites that catalyze the dehydrogenation of propane (activation step) to alkene. Comparing the mechanism of aromatization of propane on acid ZSM-5 and Zn/ZSM-5 (bifunctional catalysts). Zinc prevents the hydride combining with the alkenes to form alkane that is minimized backward hydrogenation reaction. So, this aided the formation of more aromatics [6, 9, 10, 24].
Addition of Fe as stabilizing metal increased the activity of the catalysts, which were improved to reasonable extent thereby increasing the conversion of propane significantly. Thus the conversion of the propane was sustained for a longer period of time on stream because the energy level of zinc at the reaction temperature was increased via electron mobility, Zn–Fe bimetallic compounding and zinc–iron interaction on ZSM-5 which was determined to enhance its stability. The higher activity in propane conversion after the addition of zinc and iron can be attributed to the increase in dehydrogenation activity due to the presence of Zn–Fe bridge species in the H-ZSM-5 channels. This was clearly shown in XPS and H2-TPR by increase in binding energy and reduction temperature as a result of interaction between the Zn and Fe species [23, 32, 35]. Also the dehydrogenating effect of zinc metal on zeolite by recombining hydrogen thus suppressing cracking (Beta-scission), minimizing hydrogenation backward reaction and enhancing oligomerization cyclization was enhanced by iron co-impregnation as stabilizing metal over 10 h time on stream. Zinc instability and deactivation was corrected by sustained and conversion when co-impregnated with 1–3 wt% iron loading on Zn/ZSM-5. The aromatic yield on Zn–Fe/ZSM-5 catalyst was improved and sustained as compared to only Zn/ZSM-5 (Fig. 9).
The product distributions from propane aromatization using various catalysts prepared and characterized were presented in Figs. 10, 11, 12, 13 and 14. From Fig. 10, the cracking via hydride transfer was obvious as more of lighter alkanes and cyclohexane and lower aromatic compounds were formed [25]. The high dehydrogenating effect of zinc species on HZSM-5 was clearly observed as aromatic products greatly increased with reduced alkane formation in Fig. 11, which included light gases and cyclohexane. Co-impregnation effect of Zn with 1–3 wt% Fe on ZSM-5 was clearly observed in the sustained aromatic product distributions over 10 h time on stream. In general, light alkanes, cyclohexane and C9+ were greatly reduced in the product distributions in Figs. 12, 13 and 14 as zinc stability, activity and aromatic yield were improved upon thus making more active sites available for dehydrogenation. 1 wt% Fe with Zn produced more ethylbenzene and toluene but reduced benzene among other improved aromatic compounds. Improved toluene would occur via more utilization of formed alkene and methylation of benzene to form more toluene. The formation of ethylbenzene was enhanced via dehydrogenation of formed ethane, which would have further attached to benzene. 2 wt% Fe with Zn on ZSM-5 produced reduced toluene and more of m and o-xylene via methylation of toluene (Fig. 13). Similar trend was observed for 3 wt% Fe with Zn on ZSM-5 but more sustained formation of m and o-xylene (Fig. 14). The higher the Fe content in the catalyst, the more formation of m and o-xylene as observed in Figs. 13 and 14. For all the catalysts, p-xylene has little yield as compared to other aromatic products. This may result from the difficulty in the methylation of the carbon in the benzene ring to form 1,4-dimethylbenzene as compared other m and o-xylenes that were sequentially arranged as 1,2 and 1,3-dimethylbenzene respectively. The entire improved and sustained aromatic yield recorded were as a result of increased activity of Zn via co-impregnation with Fe.
Conclusion
In conclusion, catalytic aromatization of propane was carried out over HZSM-5 supported Zn–Fe/ZSM-5 bimetallic catalysts. All characterizations revealed that the crystallinity and structure of the zeolite were preserved. It was revealed that the presence of Fe as stabilizing metal enhanced catalyst activity and aromatic yield. The modification had strong influence on activity, aromatic selectivity and product distribution with minimized undesired products. Aromatization reaction path was not altered. This study provided the direct proof of the strengthening ability of the iron second metal as it was clearly shown in the improved and sustained conversion and aromatic yield even at high reaction temperatures. Furthermore, this developed catalyst will contribute to the development of zeolite based bimetallic industrial catalysts with high activity and superior stability for desired aromatic production.
References
Fechete I, Wang Y, Védrine JC (2012) The past, present and future of heterogeneous catalysis. Catal Today 189(1):2–27
Armor JN (2011) A history of industrial catalysis. Catal Today 163(1):3–9
Lindström B, Pettersson LJ (2003) A brief history of catalysis. Cattech 7(4):130–138
Bhan A, Nicholas Delgass W (2008) Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal Rev 50(1):19–151
Bhattacharya D, Sivasanker S (1996) Aromatization of n-hexane over H-ZSM-5: influence of promoters and added gases. Appl Catal A 141(1–2):105–115
Dauda IB, Yusuf M, Gbadamasi S, Bello M, Atta AY, Aderemi BO, Jibril BY (2020) Highly selective hierarchical ZnO/ZSM-5 catalysts for propane aromatization. ACS Omega 5(6):2725–2733
Rane N, Overweg AR, Kazansky VB, van Santen RA, Hensen EJM (2006) Erratum to “Characterization and reactivity of Ga+ and GaO+ cations in zeolite ZSM-5” J. Catal. 239 (2) (2006) 478–485]. J Catal 1(240):85
Song Y, Xu Y, Suzuki Y, Nakagome H, Zhang ZG (2014) A clue to exploration of the pathway of coke formation on Mo/HZSM-5 catalyst in the non-oxidative methane dehydroaromatization at 1073 K. Appl Catal A 482:387–396
Guisnet M, Gnep NS, Aittaleb D, Doyemet YJ (1992) Conversion of light alkanes into aromatic hydrocarbons: VI. Aromatization of C2–C4 alkanes on H-ZSM-5—reaction mechanisms. Appl Catal A Gen 87(2):255–270
Choudhary TV, Kinage A, Banerjee S, Choudhary VR (2006) Influence of Si/Ga and Si/Al ratios on propane aromatization over highly active H-GaAlMFI. Catal Commun 7(3):166–169
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319
Lippens BC, Linsen BG, De Boer JH (1964) Studies on pore systems in catalysts I. The adsorption of nitrogen; apparatus and calculation. J Catal 3:32–37
Liu J, Jiang G, Liu Y, Di J, Wang Y, Zhao Z, Liu J (2014) Hierarchical macro-meso-microporous ZSM-5 zeolite hollow fibers with highly efficient catalytic cracking capability. Sci Rep 4:7276
Chu N, Yang J, Li C, Cui J, Zhao Q, Yin X, Wang JJ (2009) An unusual hierarchical ZSM-5 microsphere with good catalytic performance in methane dehydroaromatization. Microporous Mesoporous Mater 118(1–3):169–175
Sabarish R, Unnikrishnan G (2019) Synthesis, characterization and evaluations of micro/mesoporous ZSM-5 zeolite using starch as bio template. SN Appl Sci 1(9):989
Li Y, Liu S, Xie S, Xu L (2009) Promoted metal utilization capacity of alkali-treated zeolite: Preparation of Zn/ZSM-5 and its application in 1-hexene aromatization. Applied Catalysis A: General 360(1):8–16
Wannapakdee W, Suttipat D, Dugkhuntod P, Yutthalekha T, Thivasasith A, Kidkhunthod P, Wattanakit C (2019) Aromatization of C5 hydrocarbons over Ga-modified hierarchical HZSM-5 nanosheets. Fuel 236:1243–1253
Van der Borght K, Galvita VV, Marin GB (2015) Ethanol to higher hydrocarbons over Ni, Ga, Fe-modified ZSM-5: Effect of metal content. Applied Catalysis A: General 492:117–126
Liu H, Shen W, Bao X, Xu Y (2005) Methane dehydroaromatization over Mo/HZSM-5 catalysts: the reactivity of MoCx species formed from MoOx associated and non-associated with Brönsted acid sites. Appl Catal A 295(1):79–88
Waziri AY, Osigbesan AA, Dabai FN, Shuwa SM, Atta AY, Jibril BY (2019) Catalytic reforming of gaseous products from pyrolysis of low-density polyethylene over iron-modified ZSM-5 catalysts. Appl Petrochem Res 9(2):101–112
Abdelsayed V, Shekhawat D, Smith MW (2015) Effect of Fe and Zn promoters on Mo/HZSM-5 catalyst for methane dehydroaromatization. Fuel 139:401–410
El-Shall M, Abdelsayed V, Abd El Rahman SK, Hassan H, El-Kaderi HM, Thomas ER (2009) J Mater Chem 19(41):7625–7631
Jia Y, Wang J, Zhang K, Feng W, Liu S, Ding C, Liu P (2017) Promoted effect of zinc–nickel bimetallic oxides supported on HZSM-5 catalysts in aromatization of methanol. J Energy Chem 26(3):540–548
Mohiuddin E, Mdleleni MM, Key D (2018) Catalytic cracking of naphtha: the effect of Fe and Cr impregnated ZSM-5 on olefin selectivity. Appl Petrochem Res 8(2):119–129
Biscardi JA, Meitzner GD, Iglesia E (1998) Structure and density of active Zn species in Zn/H-ZSM5 propane aromatization catalysts. J Catal 179(1):192–202
Chen C, Ji H, Zhang Q, Li C, Shan H (2015) Effect of γ-alumina as active matrix added to HZSM-5 catalyst on the aromatization of methanol. Appl Petrochem Res 5(4):231–243
Mhamdi M, Ghorbel A, Delahay G (2009) Influence of the V+ Mo/Al ratio on vanadium and molybdenum speciation and catalytic properties of V-Mo–ZSM-5 prepared by solid-state reaction. Catal Today 142(3–4):239–244
Ahmad M, Farhana R, Raman AAA, Bhargava SK (2016) Synthesis and activity evaluation of heterometallic nano oxides integrated ZSM-5 catalysts for palm oil cracking to produce biogasoline. Energy Convers Manag 119:352–360
Zhou W, Liu J, Wang J, Lin L, Zhang X, He N, Guo H (2019) Enhancing propane aromatization performance of Zn/H-ZSM-5 zeolite catalyst with Pt promotion: effect of the third metal additive-Sn. Catal Lett 149(8):2064–2077
Kosinov N, Wijpkema AS, Uslamin E, Rohling R, Coumans FJ, Mezari B, Hensen EJ (2018) Confined carbon mediating dehydroaromatization of methane over Mo/ZSM-5. Angew Chem Int Ed 57(4):1016–1020
Mageed AK, Dayang ABR, Salmiaton A, Shamsul I, Musab A (2018) J King Saud Univ Sci
Dao TKT, Luu CL (2015) n-Hexane hydro-isomerization over promoted Pd/HZSM-5 catalysts. Adv Nat Sci Nanosci Nanotechnol 6(3):035014
Yan Y, Jiang S, Zhang H, Zhang X (2015) Preparation of novel Fe–ZSM-5 zeolite membrane catalysts for catalytic wet peroxide oxidation of phenol in a membrane reactor. Chem Eng J 259:243–251
Lubango LM, Scurrell MS (2002) Light alkanes aromatization to BTX over Zn–ZSM-5 catalysts: enhancements in BTX selectivity by means of a second transition metal ion. Appl Catal A 235(1–2):265–272
Yang YC, Weng HS (2009) The role of H2 in n-butane isomerization over Al-promoted sulfated zirconia catalyst. J Mol Catal A Chem 304(1–2):65–70
Roy M, Ghosh S, Naskar MK (2015) Solvothermal synthesis of Cr2O3 nanocubes via template-free route. Mater Chem Phys 159:101–106
Niu X, Gao J, Miao Q, Dong M, Wang G, Fan W, Wang J (2014) Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater 197:252–261
Sapawe N, Jalil AA, Triwahyono S, Sah RNRA, Jusoh NWC, Hairom NHH, Efendi J (2013) Electrochemical strategy for grown ZnO nanoparticles deposited onto HY zeolite with enhanced photodecolorization of methylene blue: effect of the formation of SiOZn bonds. Appl Catal A 456:144–158
Xiao H, Zhang J, Wang X, Zhang Q, Xie H, Han Y, Tan Y (2015) A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal Sci Technol 5(8):4081–4090
Migliori M, Aloise A, Catizzone E, Caravella A, Giordano G (2017) Simplified kinetic modeling of propane aromatization over Ga-ZSM-5 zeolites: comparison with experimental data. Ind Eng Chem Res 56(37):10309–10317
Montes A, Giannetto G (2000) A new way to obtain acid or bifunctional catalysts: V. Considerations on bifunctionality of the propane aromatization reaction over [Ga, Al]-ZSM-5 catalysts. Appl Catal A Gen 197(1):31–39
Acknowledgements
The authors would like to appreciate Petroleum Technology Development Fund, PTDF, for the fund provided through its annual research grant competition for the execution of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oseke, G.G., Atta, A.Y., Mukhtar, B. et al. Highly selective and stable Zn–Fe/ZSM-5 catalyst for aromatization of propane. Appl Petrochem Res 10, 55–65 (2020). https://doi.org/10.1007/s13203-020-00245-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-020-00245-9