Abstract
The gas condensate is one of the most valuable products of gas refineries. In unit 700 of the Sarkhon gas refinery, first, the gas condensate is separated from the feed. Then, the vapor pressure of the gas condensate is stabilized by de-ethanizer and de-butanizer towers. The H-701 and H-702 furnaces act as reboilers of the towers. In this research, unit 700 is simulated by HYSYS software. The product of this unit is examined to achieve desirable conditions. In addition, the best conditions are obtained to reduce the gas loss in this unit. The desirable conditions are introduced according to the operational problems of this unit. In this study, the environmental and economic loss due to the loss of hydrocarbons from the de-butanizer tower is identified. Results of this research show that the best operating temperature and pressure of the first feed are 40 °C and 29 bar, respectively. Also, the best temperature and pressure of the second feed are 20 °C and 28 bar, respectively. Also, the best temperature and pressure of the output stream from the S-701 as a feed of the T-701 are 34.35 °C and 22.51 bar, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevention of energy loss and reduction in outlet polluted gases from industries is a serious problem. In general, chemical industries are facing many operational problems in the sections of monitoring and control (Katebah et al. 2020). In addition, extracted natural gas from gas reservoirs contains a significant volume of gas condensate. Large amounts of gas condensate are wasted in different seasons of the year due to ambient temperature changes (Abdul Qyyum et al. 2022). Unlike butane and propane, gas condensate does not need special conditions to remain liquid and can be converted into light oil, gasoline, jet fuel, etc., easily (Farahbod and Karazhian 2021a, b). The surveys show that the main components of gas condensate are methane, ethane and other light hydrocarbons (Jiang et al. 2019; Maasakkers et al. 2022). The gas condensate is converted into a gas phase and is wasted in the burner if operating conditions are not suitable (Barbosa et al. 2019). In this case, the quality of the product is reduced and storage and transportation processes are disrupted. The release of light hydrocarbons endangers human health and the environment. Also, significant amounts of hydrocarbons with high economic value are wasted (Jokar et al. 2021; Farahbod and Afkhami Karaei 2021a). Therefore, gas condensate must be a stable single-phase liquid to achieve the desired conditions for storage, transfer and sale. Until now, research have been focused on the problems of gas processing, the methods of prevention of product reduction with regard to environmental aspects and energy consumption (Masnadi et al. 2020). Khalili et al. conducted research on various types of control systems to increase the quality of output gas from gas condensate stabilization unit. (Khalili-Garakani et al. 2022). In addition, another research by Zhang was conducted to increase the efficiency of gas condensate stabilization units. The results of this study showed that nonlinear control systems play a more effective role in measuring operating conditions than other control systems (Zhang and Lau 2022). The emission of exhaust gases, especially carbon dioxide, from natural gas refinery flares is one of the problems that must be evaluated by researchers (Yáñez et al. 2022; Farahbod 2022). Therefore, a study by Snytnikov has been conducted on the evaluation of flare networks in gas processing plants (Snytnikov and Potemkin 2022). The results of modeling and flame heat calculation showed that the amount of carbon dioxide emission changes with the change in flame temperature (Sarkar et al. 2022). Other scientists focused on the simulation of a gas condensate stabilization unit with the objective of minimal water vapor production (Jukić et al. 2022). In Jukić’s research, optimal operating conditions were introduced. In another study, the sensitivity analysis, economic optimization and design of refrigerant cycles of the stabilization process were investigated. In Sleiti’s research, the use of a one-stage refrigeration cycle was introduced as the best cooling method (Sleiti et al. 2022). In addition, in Sarkari’s work, steady-state simulation of the liquid natural gas process and GTL process was investigated to determine mass and energy balance (Sarkari et al. 2022). Also, in Khalili’s study, other units of the stabilization process were investigated and finally, an optimal model was proposed for simulating the process. Other researchers studied the amount of burning and emission of greenhouse gases from the flare of the gas condensate stabilization unit (Khalili-Garakani et al. 2021).
According to the previous research, it seems necessary to provide a comprehensive study that can provide the desirable operating conditions and best composition of input feed to gas condensate stabilization unit. In the present research, the composition of the main streams of the gas stabilization unit has been optimized. Also, the operating conditions of the unit have been optimized. In this research, the amount of energy reduction has been calculated and compared with the present conditions.
Materials and methods
Description of Sarkhon and Qeshm gas refinery
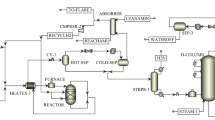
The Sarkhon and Qeshm gas refinery is a gas refinery unit located about 25 km from northeast of Bandar Abbas in Iran. This refinery works in the extraction and refining of natural gas, associated gas condensates and liquefied gas related to the Sarkhon gas field. The gas stabilization by fractionation method is used to separate light and volatile components from heavy hydrocarbons in Sarkhon and Qeshm gas refinery. The stabilization unit of the Sarkhon refinery consists of a two-phase separator (stabilizer feed drum), a stabilization tower (de-ethanizer tower (T-701)), and a reboiler at the bottom of the tower (furnace (H-701)). A stabilization tower of LPG consists of a gas condensate tower (de-butanizer tower (T-702)), a coolant at the top of the tower, a reboiler at the bottom of the tower (furnace (H-702)) and a set of heat exchangers and pumps. The feed of the de-ethanizer tower is purified through the separator (S-701) and outlet LPG from the de-butanizer tower is separated by the separator (S-702). The liquid separated from the main gas stream of unit 400 which contains gas condensate, water and glycol is sent to a three-phase separator (S-404). After separation, the output hydrocarbon as the main feed is combined with the separated hydrocarbons in separators S-205 and S-206 and sent to unit 700. The towers of unit 700 are designed to provide the needed space and time to exchange mass and energy between the liquid and vapor phases. In tray towers, the trays above the feed tray act as distillers and the trays below the feed tray act as hydrocarbons separators. The temperature of the reboiler is adjusted so that the lightest hydrocarbon in the lower part of the tower is pentane and the heaviest hydrocarbon in the gas stream above the tower is butane. The low output stream from the tower is known as the product of the stabilization unit after exchanging energy with the input feed. The part of the steam above the tower that becomes liquid in the cooling section is returned to the tower as a recycle stream to regulate the flow temperature above the tower and control the purity of the flow. Residual vapors after cooling are sent to the gas processing system as a light hydrocarbon which mainly consists of methane, ethane, propane and butane.
Description of unit 700 of Sarkhon and Qeshm gas refinery
In the Sarkhon and Qeshm refineries, hydrocarbon separation processes, dew point adjustment and gas condensate production are carried out. The produced gas condensate contains light hydrocarbons. Therefore, high pressure and low temperature are needed for the stability of the produced gas condensate. The presence of light hydrocarbons in gas condensate causes problems in transportation and storage processes. Failure to adjust the pressure in the storage tanks and the creation of two-phase flow in the transmission lines are problems that exist in the storage and transmission processes. The light hydrocarbons should be separated from gas condensates to reduce the range of vapor pressure of these products. This process is done in the stabilization unit. Light hydrocarbons in gaseous liquids are mainly methane, ethane, propane, isobutane and butane, and the amount of butane is higher than other hydrocarbons.
The stabilization of gaseous liquids of Sarkhon refinery is done through a pressure reduction process (in one step) and a distillation process (in two steps). The separation of gaseous hydrocarbons in the stabilization unit depends on the operating conditions of the distillation process. A larger amount of methane and smaller amounts of ethane, propane, butane and non-carbon compounds such as nitrogen and carbon dioxide are separated in the form of gas due to the decrease in pressure of the expansion tank. Separation of ethane from gaseous liquids is done using the distillation process in the de-ethanizer tower. The outflow from the top of the de-ethanizer tower contains a significant amount of methane gas. Propane and butane of gas liquids are separated using the distillation process and recycle stream in the de-butanizer tower. Finally, the flow of liquefied gas as a product leaves from the top of the distillation tower. Figure 1 shows the gas condensate stabilization unit.
Research method
Simulation of unit 700 of Sarkhon refinery
The gas condensate stabilization unit (unit 700) has been simulated by HYSYS software (version V 12.1) to reduce liquefied gas loss in the Sarkhon gas refinery. The HYSYS software covers a wide range of oil and gas processes. This software can model various chemical processes in steady and un-steady states with high accuracy. For simulation, the characteristics of the streams sent to the gas–liquid stabilization unit are provided. Therefore, the operating conditions and composition of each of the streams are considered input data. In the next step, the operating conditions of the incoming streams to the gas condensate stabilization unit have been changed. The iterations of input data are done with the aim of maximum convergence. Finally, the obtained results are compared with the actual conditions of the unit.
The new operational conditions of the unit have been determined and simulated to minimize the liquid gas loss and the minimum amount of environmental pollution. Ultimately, other chemical-physical properties of each stream have been evaluated after determination of the favorable operating conditions.
Description of equation of state
All of the thermodynamic equations are checked for simulations of the process. The best thermodynamic model for calculations and simulation of units is Peng-Robinson-Stryjek-Vera (PRSV) model. This is a twofold modification of the PR equation of state that extends the application of the original PR method for moderately non-ideal systems. It provides a better pure component vapor pressure prediction as well as a more flexible mixing rule than Peng Robinson. Expression 1 shows the Peng-Robinson equation of state. Equations 2 and 3 show the parameters of the Peng-Robinson formula.
\(T_{r} is\;reduced\;temperature\;and\;is\;defined\;as\,\frac{T}{{T_{c} }}.\) In addition, \(a and b are Peng-Robinson constants.\) The parameters of a and b are defined as Eqs. 4 and 5.
The intermolecular attraction parameter is modified in the Peng-Robinson equation of state. Finally, the Peng-Robinson-Stryjek-Vera (PRSV) equation of state is obtained. The attraction parameter is presented in Eqs. 6 and 7.
The Aspen-HYSYS provides an improved equation of state PRSV for rigorous treatment of hydrocarbon systems. This equation can be applied in semi-experimental and vapor pressure models for heavier hydrocarbon systems. Vapor correlations provide accurate predictions of vapor properties and activity coefficient models of chemical systems. So, this equation is used for calculations of the process in this study state. The PRSV uses an empirical factor, Kappa, for fitting pure component vapor pressures.
Advantages of PRSV equation
The advantage of the PRSV equation is that not only has the potential to more accurately predict the phase behavior of hydrocarbon systems but, it can also be used to control non-ideal systems with high accuracy. The only compromise is increased computational time and the additional interaction parameter that is required for the equation. The PR and PRSV equations of state perform three-phase flash calculations for aqueous systems containing water, methanol or glycols, as well as systems containing other hydrocarbons or non-hydrocarbons in the second liquid phase. This equation can be used in a wide range of temperatures and pressures. This equation can be applied for one, two and three phases of hydrocarbon. For these reasons, the PRSV equation is used in this research. The used model has been modified by HYSYS software and is much more accurate than the conventional model.
Tolerance value
Measurements are always associated with a certain degree of uncertainty. An efficient simulation and calculation are required to achieve high-precision measurement in the presence of uncertainty. The statistical definition of the accuracy of any measurement is defined as a standard deviation divided by the square root of the sample size taken for measurement. Accordingly, tolerance limits are statistical. Therefore, measurements should be repeated many times to achieve better accuracy. In other words, the goal of this research is to determine tolerance limits in case of uncertainty in computer and communication systems. For this simulation, a convergence criterion of one thousandth has been considered.
Investigation of stream number 1, sent to the stabilization unit
Two streams called Feed 1 and Feed 2 enter the stabilization unit. Both streams are a combination of hydrocarbons and gaseous liquids extracted from upstream unit 400 and unit 200. The characteristics and composition of Feed 1 are presented in Tables 1 and 2, respectively.
The operating conditions of Feed 1 and Feed 2 are presented in Tables 1 and 3, respectively. It is necessary to mention that “Basis-1” is a basic environment for data entry. The simulation process is managed in this environment.
In addition, the characteristics and composition of Feed 2 are presented in Tables 3 and 4, respectively. In this research, the operating conditions of both inlet streams to the stabilization unit are changed and the obtained results are compared with the present conditions. The determination of the new operating conditions is based on the minimum liquefied gas loss and the minimum amount of environmental pollution.
Evaluation of statistical analysis
In this study, the sensitivity analysis is performed to show the effect of operating conditions on target variables. Optimal operating conditions are obtained by using sensitivity analysis. In this case, one parameter is changed and other parameters are kept constant during the simulation. The optimal value of each parameter can be obtained, finally. As shown in Table 5, the sensitivity of each of the variables on the response has been calculated. The p values and R-Squared values of the process are analyzed and validated according to the obtained results. The p values and R-squared values are shown in Table 5. The lowest amount of p values shows the highest effectiveness of the variable on the target function. The results with a p value lower than 0.0001 are significant. The p values of pressure, temperature and the composition of feed indicate a significant contribution with a high value of confidence percent.
Table 5 shows the effect of major parameters on the main response of the process. The results show that the effect of recycle stream is less important than the operating conditions and the composition of the input feed to the unit. Finally, the p values and R-squared values of the stabilization process have been calculated. In this research, the operating temperature of the feed was changed between 15 and 45 °C. In addition, the operating pressure of the feed was varied between 15 and 32 bar.
Results and discussion
The best operating temperature and pressure have been obtained using the simulation of unit 700. Table 6 compares the new operating conditions with the present operating conditions.
Investigation of the composition of stabilization unit streams
In this section, the composition of streams of stabilization unit is presented in case of application of best operating conditions, as well as in case of continuation of the present conditions.
Investigation of stream number 2, output from S-701
In this section, the composition of the output stream from S-701 and input to the fuel gas unit in the present and optimal operating conditions is presented. Table 7 compares the obtained results.
Results show that the amount of methane in the output stream from S-701 increases by about 3.5% if the best conditions are applied. But, the number of compounds heavier than methane as well as carbon dioxide decreased. Table 7 shows that only the amount of nitrogen in desirable conditions is higher than present conditions. Results state that the application of desirable conditions can have an acceptable effect on the performance of the S-701. Table 7 shows that the flash process is performed better in optimal conditions. For this reason, the number of compounds heavier than methane decreases in the output stream from the S-701.
Investigation of stream number 3, input to T-701
In this section, the composition of the input stream to the T-701 in the present and desirable conditions is presented.
Table 8 shows that the amount of components such as methane, ethane, propane and iso-butane increases if the best temperature and pressure are applied by an average of 8.6%. But, the amount of hydrocarbons heavier than isobutane decreases. Meanwhile, the amount of nitrogen and carbon dioxide in desirable conditions increases by about 26 and 5%, respectively. The obtained results emphasize the necessity of the operating conditions changing. Table 8 shows that the distribution coefficient of hydrocarbons in the gas phase and liquid phase of the S-701 is improved in optimal conditions. For this reason, more light hydrocarbons are removed from this separator. Figure 2 shows the composition of the input stream to the T-701. Figure 2 shows that amount of light compounds in this flow increases by about 12% in the presence of favorable operating conditions.
Investigation of the exit stream from the top of the T-701
In this section, the composition of the output stream from de-ethanizer tower in both present and favorable conditions is presented. Table 9 provides a comparison between the composition of this stream in present and desirable conditions.
Table 9 shows that the amount of methane in the exit stream from de-ethanizer tower increases by about 5.7. Table 9 shows that other hydrocarbons and carbon dioxide in the outlet stream from the de-ethanizer tower have a decreasing trend. Meanwhile, the amount of nitrogen in the outlet stream from the de-ethanizer tower in desirable conditions is about 15.3% higher than the present conditions. The results of Table 9 show that the flow profile in T-701 is developed in optimal operating conditions and the mass transfer coefficient increases. Finally, the mass flux increases. Therefore, less heavy hydrocarbons leave T-701. In this case, more ethane is separated. Figure 3 shows that if there are optimal operating conditions, the amount of methane is about 5.2% more than the present operating conditions. This is while the amount of ethane, propane and butane decreases by 13% in optimal operating conditions.
Investigation of stream number 4, input to T-702
In this section, the composition of the input stream to the de-butanizer tower is presented. As can be seen, Table 10 provides a comparison between the composition of the inlet stream to the de-butanizer tower in present and desirable conditions.
Table 10 shows that the amount of ethane, propane, isobutane, normal butane, isopentane and normal pentane increases by about 18.9% if the present conditions change to desirable conditions. As shown in Table 10, the amount of methane in both conditions is zero. Table 10 shows that the number of hydrocarbons heavier than normal pentane in desirable conditions is less than the present conditions. In addition, results show that the amount of nitrogen and carbon dioxide in both cases is zero. Table 10 shows that the tower trays are closer to the equilibrium state and the efficiency of the tower increases in optimal operating conditions. Finally, a lighter feed enters the T-701 in the new conditions. Figure 4 shows that the amount of methane and ethane in both operating conditions is almost insignificant. But the amount of propane and butane in optimal operating conditions is about 2.1% more than the current operating conditions.
Investigation of stream number 6, output from T-702
In this section, the composition of the output stream from the bottom of the T-702 in present and desirable operating conditions is investigated. Table 11 shows the composition of this stream in present and favorable conditions. Stream number 6 is sent to the gas NGL production unit.
Table 11 shows that there is no methane and ethane in this stream. Also, the amount of propane remains constant in both cases. However, the amount of iso-butane, normal butane, iso-pentane, normal pentane and normal hexane increases by about 3% in desirable conditions. But, compounds heavier than normal hexane are reduced by about 2% in desirable conditions. The results presented in Table 11 show the better performance of the trays in optimal conditions. In other words, the mass transfer operation and the de-butanization process have been carried out better in optimal conditions. Figure 5 shows that the amount of iso-pentane and normal pentane in optimal operating conditions is about 27% more than current operating conditions. Figure 5 shows that the amount of iso-pentane and normal pentane in optimal operating conditions is about 27% more than current operating conditions.
Investigation of the through stream from PCV 714, output from S-702
In this section, the composition of the output stream from S-702 with the aim of production of LPG in present and desirable operating conditions is presented.
Table 12 shows that the output stream from S-702 is free of methane and the number of components such as ethane, propane, isobutane and normal butane in desirable conditions is higher than the present conditions. However, the output stream from this separator in both cases is free of heavy hydrocarbons, nitrogen and carbon dioxide. The obtained results confirm the need to change the present operating conditions. Table 12 shows that the phase changing and volatility of hydrocarbons with average molecular weight are improved in optimal operating conditions, slightly. Therefore, the amount of ethane, propane, isobutane, and normal butane in the output stream from S-702 increases in optimal operating conditions, slightly. Figure 6 shows the amount of propane and iso-butane in optimal operating conditions and current operating conditions. The simulation results show that the amount of propane and iso-butane in optimal conditions is about 7.2% higher than the current conditions.
Investigation of energy consumption of stabilization unit
Providing energy for gas condensate stabilization units and liquefied gas production requires high costs. Therefore, the reduction in energy consumption in this unit is one of the most important factors that can save costs. In this section, energy consumption can be evaluated after the reduction in gas loss and increasing the quality of LPG. In other words, the composition of the produced LPG becomes favorable with the improvement of the operating conditions. Finally, the amount of wastage decreases. Table 13 provides a comparison between the energy consumption of the major equipment of this unit in present and desirable conditions.
Table 13 states that the energy required for stabilization unit equipment is reduced significantly in favorable conditions. Therefore, the operating costs of this unit are reduced, significantly. Table 13 shows that the energy consumption decreases by about 0.21% if the stabilization unit operates in desirable conditions. Table 13 shows that the amount of energy consumed by the main equipment of the process decreases if the stabilization process works in optimal conditions. Figure 7 shows that in the presence of optimal conditions, the energy consumption of the equipment decreases. As a result, the amount of total energy is reduced by about 0.5%.
Introduction of desirable conditions of the stabilization unit
Table 14 shows the values of temperature and pressure of the main streams of the stabilization unit in present and favorable conditions.
Conclusions
(1) The best composition of the input stream to de-butanizer tower is presented in this research.
(2) The desirable composition of input/output streams and the best composition of streams are presented in this work.
(3) The energy consumption of the basic equipment of the stabilization unit and the amount of natural gas and liquefied gas loss in desirable conditions are presented in this research.
(4) This study shows that the best temperature and pressure of the second input feed to the stabilization unit are 20 °C and 28 bar, respectively.
(5) Simulation results show that the appropriate operating temperature and pressure for the NGL and LPG units are equal to 60.53 °C and 2.5 bar, as well as 55.65 °C and 16.7 bar, respectively.
Data availability
Not applicable.
Abbreviations
- E:
-
Exchanger
- GTL:
-
Gas to Liquid
- H:
-
Heater
- Liq.:
-
Liquid
- LPG:
-
Liquefied Petroleum Gas
- NGL:
-
Natural Gas Liquid
- P:
-
Pump
- PCV:
-
Pressure Control Valve
- PRSV:
-
Peng-Robinson-Stryjek-Vera
- S:
-
Separator
- Std.:
-
Standard
- T:
-
Tank
- V:
-
Vessel
- Vol.:
-
Volume
- a:
-
Peng-Robinson constant \((\frac{Pa{.m}^{6}}{{grmol}^{2}})\)
- b:
-
Peng-Robinson constant \((\frac{{m}^{3}}{grmol})\)
- P:
-
Operating pressure \((Pa)\)
- \({P}_{c}\) :
-
Critical pressure \((Pa)\)
- \({P}_{r}\) :
-
Reduced pressure
- R:
-
Gas constant \((\frac{J}{grmol.K})\)
- T:
-
Operating temperature
- \({T}_{c}\) :
-
Critical temperature \((K)\)
- \({T}_{r}\) :
-
Reduced temperature
- V:
-
Volume \((\frac{{m}^{3}}{mol}\))
- \(\alpha\) :
-
Correction factor
- \(\kappa\) :
-
Attraction parameter
- \(\omega\) :
-
Acentric factor
References
Abdul Qyyum M, Naquash A, Haider J, Al-Sobhi SA, Lee M (2022) State-of-the-art assessment of natural gas liquids recovery processes: techno-economic evaluation, policy implications, open issues, and the way forward. Energy 238:121684
Barbosa LC, Araújo OQF, Medeiros JL (2019) Carbon capture and adjustment of water and hydrocarbon dew-points via absorption with ionic liquid [Bmim][NTf2] in offshore processing of CO2-rich natural gas. J Nat Gas Sci Eng 66:26–41
Farahbod F (2022) Evaluation of reducing CO2 emissions as important greenhouse gas and maximum oil recovery: optimization of two processes. Int J Env Sci Tech 18(7):1821–1836
Farahbod F, Afkhami Karaei M (2021a) Mathematical modeling and experimental study of sulfur removal process from light and heavy crude oil in a bed occupied by ferric oxide nanocatalysts. Env Tech Innov 23:101656
Farahbod F, Karazhian N (2021b) Presentation of Farahbod-Karazhian equation as an accurate mathematical model based on thermodynamics and fluid flow with the aim of predicting the deposition rate of oil heavy compounds in heat exchangers. Energy Source Part a: Recovery Util Environ Eff. https://doi.org/10.1080/15567036.2021.1955047
Jiang H, Zhang Sh, Jing J, Zhu C (2019) Thermodynamic and economic analysis of ethane recovery processes based on rich gas. App Therm Eng 148:105–119
Jokar SM, Wood DA, Sinehbaghizadeh S, Parvasi P, Javanmardi J (2021) Transformation of associated natural gas into valuable products to avoid gas wastage in the form of flaring. J Nat Gas Sci Eng 94:104078
Jukić L, Vulin D, Lukić M, Sedlar DK (2022) Enhanced gas recovery and storability in a high CO2 content gas reservoir. Int J Green Gas Cont 117:103662
Katebah MA, Hussein MM, Shazed AR, Bouabidi Z, Al-musleh EI (2020) Rigorous simulation, energy and environmental analysis of an actual ba seload LNG supply chain. Comp Chem Eng 141:106993
Khalili-Garakani A, Iravaninia M, Nezhadfard M (2021) A review on the potentials of flare gas recovery applications in Iran. J Clean Prod 279:123345
Khalili-Garakani A, Nezhadfard M, Iravaninia M (2022) Enviro-economic investigation of various flare gas recovery and utilization technologies in upstream and downstream of oil and gas industries. J Clean Prod 346:131218
Maasakkers JD, Omara M, Gautam R, Lorente A, Pandey S, Tol P, Borsdorff T, Houweling S, Aben I (2022) Reconstructing and quantifying methane emissions from the full duration of a 38-day natural gas well blowout using space-based observations. Rem Sen Env 270:112755
Masnadi MS, Perrier PR, Wang J, Rutherford J, Brandt AR (2020) Statistical proxy modeling for life cycle assessment and energetic analysis. Energy 194:116882
Sarkar S, Quddus N, Mannan MS, El-Halwagi MM (2022) Integrating flare gas with cogeneration system: Hazard identification using process simulation. J Loss Prev Proc Ind 74:104635
Sarkari M, Jamshidi B, Ahmadi Khoshooei M, Fazlollahi F (2022) Flare gas reduction: a case study of integrating regeneration gas in flash gas compression network. Fuel 318:123661
Sleiti AK, Al-Ammari WA, Aboueata KM (2022) Flare gas-to-power by direct intercooled oxy-combustion supercritical CO2 power cycles. Fuel 308:121808
Snytnikov P, Potemkin D (2022) Flare gas monetization and greener hydrogen production via combination with cryptocurrency mining and carbon dioxide capture. Isci 25(2):103769
Yáñez É, Meerman H, Ramírez A, Castillo É, Faaij A (2022) Fully integrated CO2 mitigation strategy for an existing refinery: a case study in Colombia. App Energy 133:118771
Zhang K, Lau HC (2022) Utilization of a high-temperature depleted gas condensate reservoir for CO2 storage and geothermal heat mining: a case study of the Arun gas reservoir in Indonesia. J Clean Prod 343:131006
Funding
No funds have been received from this body or organization or country to conduct this research.
Author information
Authors and Affiliations
Contributions
I am the only author of this research and I have done all the work alone up to this point.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
I am the only author of this research and I have done all the work alone up to this point. If I have to use other people's knowledge during the revision process, I can act freely in all cases.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farahbod, F. Improvement of operating conditions and composition of streams in gas condensate stabilization unit with the aim of reducing flare gas in Sarkhon and Qeshm gas plant. J Petrol Explor Prod Technol 14, 555–566 (2024). https://doi.org/10.1007/s13202-023-01718-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01718-y